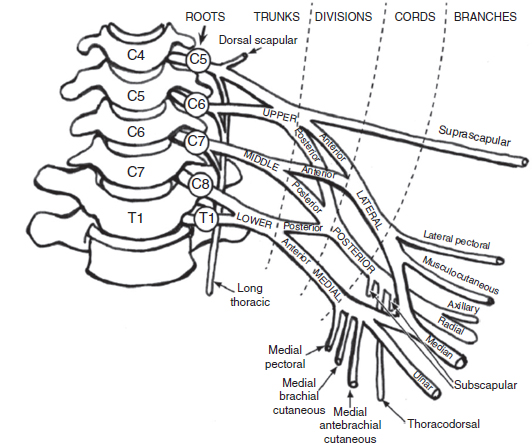
10 Peripheral nerve injury can occur at any age and as such can involve a variety of structures including the brachial plexus. Brachial plexopathy is a general term used to indicate injury to the brachial plexus. The term does not specify the cause of the injury, and the etiology could be traumatic, vascular, inflammatory, or other. It also does not specify the specific anatomic area of the brachial plexus that is involved. However, of all of the plexopathies, brachial plexopathies are the most common and represent a variety of disorders. Electrodiagnostic testing is an important component in the clinical assessment of brachial plexopathies and can provide accurate localization of the brachial plexus regions affected and shed light on the possible pathophysiologic processes involved. Recognized as an extension of the neurologic examination, electrodiagnostic testing can provide objective evidence of specific muscle involvement that is not always clinically obvious, provide objective confirmation of abnormal muscles identified on physical examination, show active denervation and reinnervation changes in affected muscles, identify patterns consistent with demyelinating and axonal pathology, estimate severity of the pathology, and also provide for the possibility of serial examinations over time to objectively assess clinical progress and help with guiding management and establishing prognosis. Electrodiagnostic testing of the brachial plexus can be a complicated and difficult task precisely because of its complicated anatomy and anatomic location. It is considered to be the most complex peripheral nervous system structure, and its superficial location, relatively large size, and position between the neck and upper extremity places it at risk for a variety of disorders, especially trauma. In order to be able to perform accurate and comprehensive electrodiagnostic testing, a clear understanding of the regional and brachial plexus anatomy is essential. In addition, a working knowledge of the disorders affecting the brachial plexus is helpful in formulating an electrodiagnostic interpretation of the findings. When a patient is referred for electrodiagnostic testing of possible brachial plexopathy, the electromyographer can use the tools available, such as sensory and motor nerve conduction studies (NCSs) in conjunction with needle electromyographic (EMG) examination to distinguish between a radiculopathy, plexopathy, a more distal neuropathy, and mononeuropathy multiplex. In the case of a brachial plexopathy, electrodiagnostic testing can localize the lesion within the brachial plexus. In order to be able to do this, the electromyographer must have an excellent understanding of the brachial plexus anatomy. The following will provide an overview of the anatomy of the brachial plexus. It is intended to be a review relevant to electrodiagnostic assessment and not a comprehensive anatomical review, which is beyond the scope of this chapter. For a detailed review of the brachial plexus anatomy, the reader is referred to Wilbourn (1). The brachial plexus is a triangular-shaped structure, which lies between two mobile structures, the neck and upper extremity. It extends from the spinal cord to the axilla, its average length is 15.3 cm, and it has a 2 to 1 ratio of connective-to-neural tissue composition (2,3). The brachial plexus is derived from the ventral roots of C5 through T1 and is sequentially divided into five components: roots, trunks, divisions, cords, and terminal nerves (Figure 10.1). There are five roots, C5 to T1; three trunks: upper, middle, and lower; six divisions: three anterior and three posterior; three cords: lateral, posterior, and medial; and several terminal branches (Tables 10.1–10.2). FIGURE 10.1 The components of the brachial plexus: roots, trunks, divisions, cords, and major terminal branches. Source: From Ref. (4). Simmons Z. Electrodiagnosis of brachial plexopathies and proximal upper extremity neuropathies. Phys Med Rehabil Clin N Am. 2013;24:13–32. TABLE 10.1 Brachial Plexus Composition The neural elements of the brachial plexus have their origin from the C5 through the T1 levels of the spinal cord. The most proximal exit the spinal cord as dorsal and ventral rootlets; they come together, forming the dorsal and ventral roots. Both dorsal and ventral roots then fuse together to form a mixed (sensory and motor fibers) spinal nerve at a point just distal to the dorsal root ganglion (DRG). After exiting the intervertebral foramen, the spinal nerve then branches posteriorly into the posterior primary rami and continues as the anterior primary rami. The anterior primary rami, considered to be the roots of the brachial plexus, are located immediately external to the intervertebral foramina and emerge from between the anterior and middle scalene muscles. In regard to the overall root contribution to the brachial plexus, each of the C6, C7, and C8 roots contributes approximately 25% of its neural elements, and the remainder is provided by the C5 and T1 roots (2). It should also be understood that the percentage of motor and sensory fiber composition of each root varies. Typically, C5 and C6 roots contain the largest percentage of motor fibers, while C7 and T1 have the least. Sensory fibers are found in greatest numbers in the C7 root, followed by C6, C8, T1, and C5, in descending order (3). As is to be expected, anatomic variation exists. “Prefixed plexus” is a term used when there is contribution from C4 and minimal contribution from T1. In these cases, nerve contributions to the brachial plexus are shifted one level superiorly (4). There is also a “postfixed plexus”; this exists when there is minimal contribution of C5 and significant contribution from T2. In this case, nerve contribution to the brachial plexus is shifted one root level inferiorly. Finally, the brachial plexus is considered expanded when root contribution comes from C4 through T2. It is important to note that there are two nerves that originate directly from the nerve roots: TABLE 10.2 Upper Extremity Nerves With Corresponding Cord Origin 1. Long thoracic nerve; origin: directly from C5, C6, and sometimes from C7 anterior primary rami; innervation: serratus anterior muscle. 2. Dorsal scapular nerve; origin: C5 root and can sometimes have contribution from C4 or C6; innervation: levator scapulae and major and minor rhomboid muscles. Important note: Clinical involvement of these muscles, in the form of weakness and/or EMG abnormalities, indicates a very proximal lesion, usually at the root level. From an EMG perspective, it is important to recall that the cervical paraspinal muscles are innervated by the posterior primary rami, indicating that their innervation comes directly from the root level. Preganglionic sympathetic fibers reach the sympathetic ganglia after exiting via the anterior primary rami and white rami communicantes. Sympathetic ganglia send postganglionic fibers to C5 through T1 spinal nerves via gray rami communicantes. The three trunks (upper, middle, and lower) are located in the posterior cervical triangle behind the clavicle and sternocleidomastoid. The upper trunk is formed by the union of the C5 and C6 anterior primary rami. The middle trunk is the continuation of the C7 anterior primary ramus, whereas the lower trunk is formed by a merger of the C8 and T1 anterior primary rami. There are two terminal nerves originating from the upper trunk: the suprascapular nerve and the nerve to the subclavius muscle. No significant terminal branches originate from the middle or lower trunks. Progressing distally, each trunk divides into an anterior and posterior division, all located behind the clavicle. There are no terminal nerve branches that arise from the divisions. There are three cords, which are formed from the six divisions. Their names are derived from their location in relation to the course of the second segment of the axillary artery. The cords begin at or just distal to the clavicle and lie below the pectoralis major in the proximal axillary region, near the axillary lymph node chain and the major vascular structures to the arm. The cords are formed in the following manner: Several nerves arise from the cords and these are summarized in Table 10.2. The location of these neural structures is in the distal axilla and includes the median, ulnar, and radial nerves and in some descriptions, also includes the musculocutaneous and axillary nerves (3). There is no clear consensus regarding when the terminal nerves become peripheral nerves. However, this has been defined as 3 cm beyond the cord or at the point where the nerves exit the axilla (5,6). Although brachial plexopathies can arise from many causes and be classified in several ways, perhaps the best method of classification is according to the area of involvement. One method is to divide the plexopathies in the following manner: 1. Supraclavicular a. Roots b. Trunks 2. Retroclavicular a. Divisions 3. Infraclavicular a. Cords b. Terminal nerves This classification scheme has practical, clinical utility because the incidence, severity, prognosis, and lesion type vary according to the area involved (3). Supraclavicular plexus lesions are further subdivided into 1. Upper a. C5 and C6 roots b. Upper trunk i. More commonly due to demyelinating conduction block ii. Closer to the muscles innervated iii. Extraforaminal 2. Middle a. C7 root b. Middle trunk 3. Lower a. C8 and T1 roots b. Lower trunk Infraclavicular plexus lesions are not further divided because there appear to be no regional differences in lesion type, severity, incidence, and prognosis. Injury to the brachial plexus is caused by a variety of disorders similar to those of the other peripheral nervous system structures. The type of pathologic injury can be classified into two main types: axon loss and demyelination. As a result, these can produce three types of pathophysiologic changes: conduction slowing, conduction block, and conduction failure (7). In general, and regardless of the region affected, axon loss is the most common pathology encountered in brachial plexopathies. Essentially, any type of injury, if sufficiently severe, can destroy nerve fibers, regardless of size and of whether the axons are myelinated or unmyelinated. When considering focal lesions producing axonal loss, it is important to take into account the concept of Wallerian degeneration. Because the entire axonal segment of the axon distal to the lesion is separated from the cell body, it undergoes Wallerian degeneration. Obviously, this will affect all the structures innervated by those axons. Clinically, the symptoms associated with axon loss can be conceptualized as negative and positive. Negative symptoms can be thought of in terms of losing function such as weakness and atrophy if motor axons are lost. Sensory loss of all modalities occurs if sensory axons are lost. Positive symptoms of motor axon loss include fasciculations, myokymia, and cramps. Pain can be observed as a result of small-fiber sensory axon loss and paresthesia from large-fiber sensory axonal injuries. In the case of a complete axonal lesion, conduction block is present at the site, and electrical stimulation of the neural structure will yield the following: This phenomenon is present as long as the distal nerve stump is capable of impulse conduction; 6 days for motor fibers and 8 to 9 days for sensory fibers. At approximately day 11, conduction failure is present, and no motor or sensory responses are elicited due to the advanced degeneration of the distal stump. Injuries that are of a type insufficient to produce axon loss may still be sufficient to produce injury to myelinated axons, causing focal demyelination. This type of pathology is localized and does not affect other, proximal or distal, areas of the nerve segment. However, conduction slowing and conduction block can arise from this type of pathology, and the resulting conduction abnormality depends on the severity of the demyelination. Focal demyelinating conduction slowing is less severe and is further characterized by: Focal demyelinating conduction block is a much more severe condition because it blocks nerve impulses from crossing the lesion. This condition is similar to conduction failure encountered with axonal degeneration, and the clinical manifestations are essentially the same. It can involve any area of the plexus. Although in many cases it is clinically indistinguishable from conduction failure, there are three major differences between the two (Table 10.3): Brachial plexopathies are caused by a variety of conditions and these can affect any part of the brachial plexus. As such, it is useful to divide the disorders into those, which are localized to the supraclavicular region and those localized to the infraclavicular regions. Some common brachial plexus disorders, not limited to children, affecting these regions are listed in Table 10.4. TABLE 10.3 Conduction Block Versus Conduction Failure
EMG in Pediatric Brachial Plexopathy
ANATOMY
NEURAL STRUCTURE
NUMBER
COMPONENT
Roots
5
C5–T1
Trunks
3
Upper, middle, lower
Divisions
6
3 anterior, 3 posterior
Cords
3
Lateral, posterior, medial
Terminal
Several
Nerves
Roots
LATERAL CORD
MEDIAL CORD
POSTERIOR CORD
Lateral pectoral
Medial pectoral
Upper subscapular
Musculocutaneous
Medial brachial cutaneous
Lower subscapular
Lateral antebrachial cutaneous
Medial antebrachial cutaneous
Thoracodorsal
Ulnar
Axillary
Median
Radial
Trunks
Divisions
Cords
Lateral Cord
![]() Upper trunk anterior division
Upper trunk anterior division
![]() Middle trunk anterior division
Middle trunk anterior division
![]() Contains C6 to C7 sensory and C5 to C7 motor fibers
Contains C6 to C7 sensory and C5 to C7 motor fibers
![]() Has no C5 sensory fibers
Has no C5 sensory fibers
Medial Cord
![]() Lower trunk anterior division
Lower trunk anterior division
![]() C8 and T1 sensory fibers present
C8 and T1 sensory fibers present
![]() C8 and T1 motor fibers present
C8 and T1 motor fibers present
Posterior Cord
![]() Upper, middle, and lower trunk posterior divisions
Upper, middle, and lower trunk posterior divisions
![]() C5 to C7 sensory fibers present
C5 to C7 sensory fibers present
![]() C5 to C8 motor fibers present
C5 to C8 motor fibers present
![]() No C8 sensory fibers present
No C8 sensory fibers present
Terminal Nerves
Brachial Plexopathy Classification
![]() Supraclavicular are more common and
Supraclavicular are more common and
![]() Are usually due to closed traction injuries
Are usually due to closed traction injuries
![]() Tend to be more severe as greater force is required to produce these injuries
Tend to be more severe as greater force is required to produce these injuries
![]() Are associated with poor outcome; except for those involving the upper trunk from which patients tend to have better recovery
Are associated with poor outcome; except for those involving the upper trunk from which patients tend to have better recovery
PATHOPHYSIOLOGY
Axonal Degeneration and Axon Loss
![]() Proximal stimulation generates no response
Proximal stimulation generates no response
![]() Distal stimulation generates responses, which decrease in amplitude with each succeeding day
Distal stimulation generates responses, which decrease in amplitude with each succeeding day
![]() Motor fibers 2 to 3 days
Motor fibers 2 to 3 days
![]() Sensory fibers 5 days
Sensory fibers 5 days
Focal Demyelination
![]() All nerve impulses cross the lesion, but the impulse transmission velocity is reduced.
All nerve impulses cross the lesion, but the impulse transmission velocity is reduced.
![]() Weakness, atrophy, or sensory deficits are not seen because the nerve impulses are able to reach their destination.
Weakness, atrophy, or sensory deficits are not seen because the nerve impulses are able to reach their destination.
![]() It can produce positive phenomena such as fasciculations, myokymia, cramps, and paresthesias.
It can produce positive phenomena such as fasciculations, myokymia, cramps, and paresthesias.
![]() If differential slowing is present along the nerve, then nerve impulse synchrony will be affected, and this can manifest as altered vibration, position, and light touch sensation, as well as changes in muscle stretch reflexes. This occurs because these require synchronized volleys.
If differential slowing is present along the nerve, then nerve impulse synchrony will be affected, and this can manifest as altered vibration, position, and light touch sensation, as well as changes in muscle stretch reflexes. This occurs because these require synchronized volleys.
Causes of Brachial Plexopathies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






