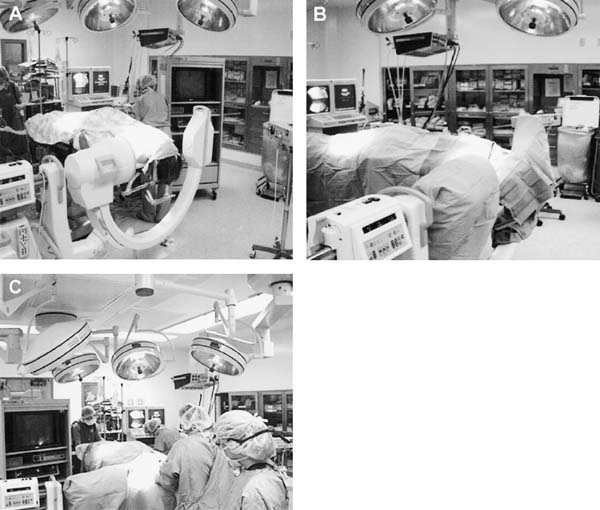
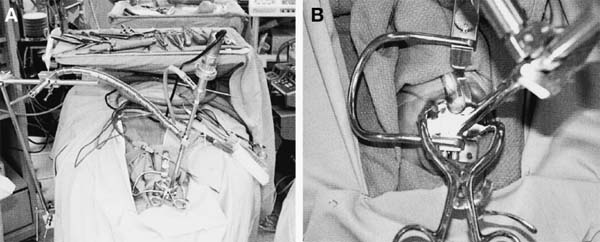
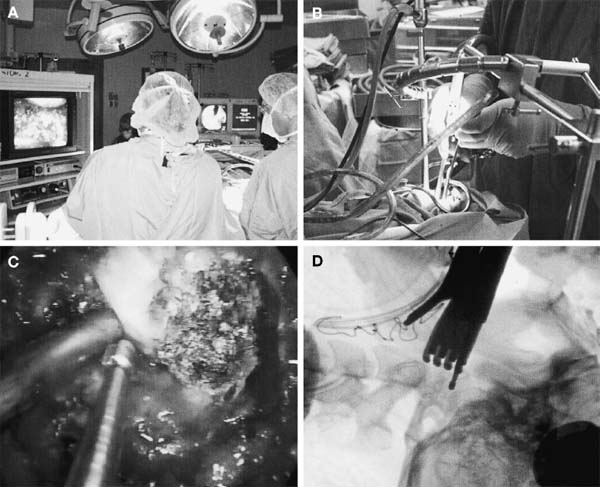
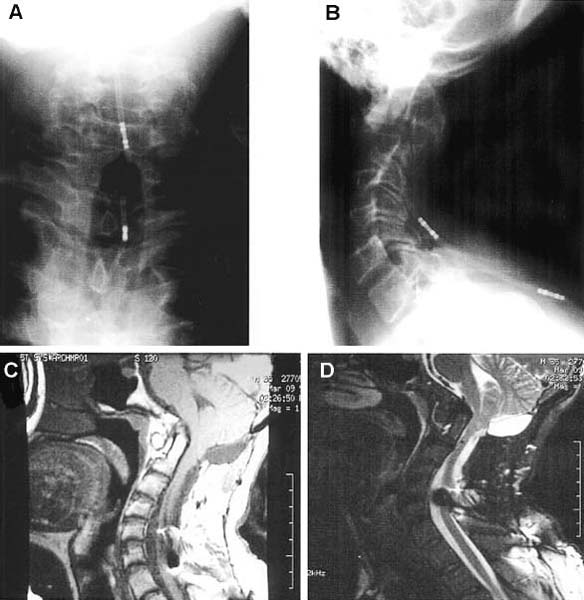
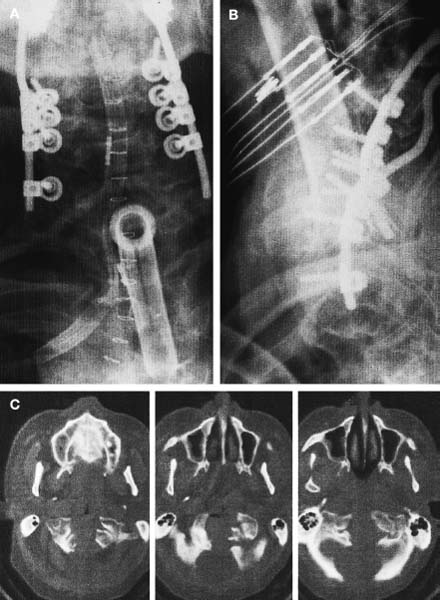
The transoral approach is commonly used to treat anterior pathological lesions in the lower clivus, foramen magnum, and upper cervical spine. It provides excellent visualization of extradural lesions that extend from the mid-clivus to the level of the C2 vertebral body. The standard microsurgical transoral technique has permitted a safe and direct approach to anterior pathology of the upper cervical spine and lower clivus with reduced mortality and morbidity.1–3 Refinements using neuronavigation have further optimized this procedure.4,5 Despite advances in microneurosurgical techniques, the standard transoral approach does have limitations. The narrow working space can limit rostral and caudal exposure. Hard palate resection and extensive maxillotomies are used to enhance cephalad visualization. Splitting the mandible and glossectomy can improve caudal exposure. In an effort to reduce morbidities associated with additional surgical exposure, surgeons have begun to explore adjuvant intraoperative techniques and equipment. In this chapter, we will describe the use of a magnifying endoscope in the transoral approach for anterior cervicomedullary junction decompression. The endoscopically assisted transoral (EATO) approach offers superior visualization and illumination compared with the standard anterior microsurgical approach to the caudal clivus and upper cervical spine. In our experience, use of the endoscope, which has a 30-degree angle, has obviated the need for extensive soft palate splitting, hard palate resection, and extended maxillotomy except in cases of very high clival pathology.6 Thus, the EATO approach represents a less-invasive means of achieving transoral decompression of the upper cervical spine and lower clivus. Indications Indications for the endoscopic-assisted and standard transoral approaches are essentially the same. Numerous anterior pathologies (e.g., extradural tumor, translocation of the odontoid process in rheumatoid arthritis, basilar impression, congenital atlantoaxial subluxation, fracture-dislocation at the craniocervical junction, vertebrobasilar aneurysm, and anterior compression of the neural structures) can be approached by the transoral route. For lesions that may require extensive additional surgical exposure to visualize rostral or caudal disease, EATO is especially indicated because additional surgical exposure may be avoided altogether. Although some authors have successfully used the transoral approach for intradural lesions, the transoral approach is not generally recommended for intradural lesions because of the risk of complications related with dural opening (e.g. cerebrospinal fluid, CSF, leak and meningitis).7 In addition, the transoral procedure is contraindicated in patients with ectatic vertebral or basilar artery located in the operative field, or if an active infectious process is present in the nasopharyngeal cavity.3 Preoperative Procedures Preoperative Assessment Preoperative evaluation of the oral cavity is extremely important before doing the transoral procedure. An interdental length of 2.5 cm is the minimum distance required for adequate exposure using the transoral route; therefore, in any patient with less than 2.5 cm of interdental space, splitting the mandible and glossectomy usually provides the additional room necessary for performing surgery. A culture of the nasal and oropharyngeal cavities is obtained preoperatively to identify unusual organisms and guides perioperative antibiotic selection. On the other hand, triple antibiosis can be used routinely. Patient Positioning The patient is positioned supine on the operating table, with the head slightly extended and fixated rigidly with a head holder (Fig. 4–1A). Rigid fixation of the head, however, is not used in some institutions to avoid potential injury during the procedure that may result from distal segment movement away from a fixed craniocervical junction.3,8 A halo brace can be used if the patient had preoperative spinal instability. Awake, fiberoptic oral intubation is indicated for patients with instability. Tracheostomy is generally reserved for cases with severe respiratory disturbance or lower cranial deficits that require prolonged postoperative ventilatory support. OR Setup and Equipment Several items of equipment are needed to perform an endoscopic transoral procedure safely. First, C-arm fluoroscopy is essential for verifying correct patient positioning and localization during surgery. The C-arm should be draped into the field (Fig. 4–1B). Some authors have further advocated the use of neuronavigation software in standard transoral approaches to reduce postoperative morbidity.4,5 At this time, it is not our practice to use image guidance for the EATO procedure. Next, a Dingman retractor with rubber guards is used to keep the tongue depressed and the mouth open and to protect the airway. The choice of endoscopes is important, and we currently use the 10- and 30-degree angled 10 mm Karl Storz endoscopes (Karl Storz Endoscopy-America, Inc., Culver City, CA). The basic surgical setup is similar to the standard transoral approach (see Fig. 4–1C). A high-speed drill (Midas Rex, Ft. Worth, TX) is required to remove bone, as well as pituitary rongeurs and curettes. FIGURE 4–1 Operating room setup. (A) The patient is placed supine, in three-point head fixation, in mild extension. (B) The patient is draped to include the fluoroscopy and to allow access to the nasal-pharyngeal cavity. (C) For convenience, anesthesia is located at the foot of the bed. The video tower is placed opposite the surgeon, and the fluoroscopy monitor is placed at the foot of the bed. (With permission from Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51:60–66.) FIGURE 4–2 The endoscopic position. (A) The endoscope is held in place via a flexible arm that is secured to the operating table. (B) The endoscope is placed in the operative field and provides illumination and magnification. (With permission from Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51:60–66.) Surgical Technique Following induction and intubation, gauze throat packs are placed to occlude the larynx and the esophagus to limit accumulation of blood in the stomach. The oropharyngeal cavity is sterilized with 10% providine iodine solution and hydrogen peroxide. The patient is then draped to allow access to the mouth and the nasal cavity (see Fig. 4–1B). A Dingman retractor is placed over the teeth and used to keep the mouth open. Self-retaining retractors are fastened to the Dingman to keep the tongue depressed. It is a good practice to release tongue retraction every 30 min to prevent lingual congestion from venous and lymphatic compression. Next, the soft palate is injected with 1% lidocaine with 1/100,000 epinephrine. With the aid of loupe magnification, a midline incision is made in the soft palate to the base of the uvula deviating to one side of it. Crockard self-retaining retractors are inserted to retract the leaves of the soft palate, allowing exposure of the high nasopharynx. The posterior pharyngeal wall is anesthetized with 1% lidocaine with 1/100,000 epinephrine. Ten- and 30-degree 10 mm Karl Storz endoscopes are used for illumination and visualization of the operative field (Fig. 4–2). A midline posterior pharyngeal incision is made that extends from the base of the clivus to the upper border of the third cervical vertebra as guided by lateral fluoroscopy. The pharyngeal flaps are elevated and retracted using a Crockard self-retaining retractor exposing the prevertebral and retropharyngeal muscles. The incision is carried down to the bone using an insulated electrocautery, and a subperiosteal dissection of the longus coli and longus capitis muscles is then done. It is important to limit the lateral exposure to 15 mm from the midline bilaterally to prevent injury to the hypoglossal nerves, eustachian tubes, and vertebral arteries at the C1–C2 interspace.6 Self-retaining retractors are placed to expose the lower clivus, C1, and C2. Using a high-speed drill under endoscopic and lateral fluoroscopic guidance, the anterior arch of C1 and the mid- to caudal clivus are removed as needed to expose the dens completely (Fig. 4–3). A large amount of pannus is frequently present between the anterior arch of C1 and the dens, especially in rheumatoid patients. This can be removed using the cautery, curettes, and pituitary rongeurs, remembering to expose no more than 15 mm lateral to the midline. The apical ligament is then incised and removed. In cases of severe odontoid invagination, the caudal clivus is resected using the high-speed drill under endoscopic and lateral fluoroscopic guidance. Care must be taken because the tissue posterior to the clivus is often adherent to the dura. Bleeding can occur if the marginal sinus is violated at the level of the foramen magnum, but this is easily controlled using Gelfoam and the bipolar cautery. FIGURE 4–3 The anatomic exposure (A) The surgeon views the decompression on the video monitor. (B) A closer view of the surgeon working in the operative field. (C) An endoscopic view of the transoral decompression. (D) Fluoroscopy of C2 decompression in progress. (With permission from Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51:60–66.) Removal of the dens is then accomplished in a rostralcaudal direction using a high-speed drill. Complete removal of the dens requires sectioning of the rostral alar and apical ligaments, which are often adherent to the dura. Sectioning of these ligaments is easier if the base of the dens is still intact and the tip is not floating.1 After bony removal, the pannus is resected. This is also accomplished in a rostral to caudal direction after identification of the dura/ligamentous plane rostrally. Decompression should be extensive, including the fibrous capsule around the odontoid and the overlying synovial tissue. Cervicomedullary decompression is judged to be complete when dural pulsations are observed at the decompression site. Adequacy of decompression is then confirmed using lateral fluoroscopy and locally applied iodinated contrast agent. Hemostasis is achieved after the decompression is completed, and a Valsalva maneuver is performed to rule out cerebrospinal fluid (CSF) leak. If the dura is opened, primary closure of the dura with a fascial graft and fat pad should be attempted. Fibrin glue can also be used to reinforce dural closure. A lumbar drain and intravenous antibiotics are maintained for 7 to 10 days following the procedure to prevent further CSF leak and fistula formation. Complications and Their Avoidance Several potential complications can be precluded by careful attention during the intraoperative and postoperative periods. These include complications of dural tears, postoperative nutritional support, and spinal stability. Dural tears ideally will be prevented by meticulous surgical dissection; however, in the event of a durotomy, optimal treatment is aimed at primary closure with a fascial graft, fat pad, or fibrin glue. Dural tears that are not recognized and corrected intraoperatively should be suspected if the patient complains of headache, nuchal tenderness, or a sensation of mucous discharge along the pharynx. A lumbar drain for 7 to 10 days with intravenous antibiotics is considered appropriate initial therapy. During the immediate postoperative period, the patient should remain intubated for 1 to 3 days while tongue swelling subsides. Topical steroid may help reduce soft tissue swelling of the oral cavity. Nutritional support is important during the immediate postoperative period and starts with feeding through a nasogastric tube. The diet can be advanced to clear liquids at the end of postoperative week 1 and then to a soft diet the following week. FIGURE 4–4 Case illustration, preoperative radiographs. (A) Anteroposterior x-rays. (B) Lateral x-ray. (C). T1-weighted sagittal magnetic resonance imaging (MRl). (D) T2-weighted MRI. (With permission from Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51:60–66.) Postoperative spinal stability should also be evaluated carefully because the odontoidectomy may create instability at the craniocervical junction. Menezes and Van Gilder3 consider all patients with rheumatoid arthritis who undergo transoral odontoidectomy as unstable. If the craniocervical junction is unstable, patients must be immobilized with a cervical orthosis, and an occipitocervical fusion needs to be performed. Some surgeons advocate doing the odontoidectomy and posterior fusion on the same day.7,9 Tuite et al10 have demonstrated successful fusion in pediatric patients who underwent perioperative posterior fusion following transoral decompression. Case Illustration A 36-year-old man with a history of type I Chiari malformation with syringomyelia presented to an outside institution complaining of neck pain and progressive difficulty with upper extremity fine motor control. He was initially treated with cervical laminectomy and syrinx-toperitoneal shunt placement. He did well initially, but he noted a gradual return of his symptoms during the course of the next year, which prompted a revision of his syrinx-to-peritoneal shunt. When this failed to improve his symptoms, he underwent posterior fossa decompression with duroplasty. He again did well but noted an 8-month history of progressive numbness extending from his head to his fingertips, with difficulty walking with frequent falls, and an inability to have or maintain erections. He also reported changes in the tone and quality of his voice and severe difficulty swallowing, prompting placement of a gastrostomy tube. FIGURE 4–5 Case illustration. Postoperative radiographs: (A) Anteroposterior x-ray. (B) Lateral x-ray. (C) Computed tomographic scan of C1 and C2 showing extent of decompression. (With permission from Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51:60–66.) CT and magnetic resonance imaging (MRI) revealed severe compression of his cervicomedullary junction caused by a posteriorly migrated dens with platybasia of the clivus, cranial settling, and occipitalization of C1. He had adequate posterior fossa decompression and a multilevel cervical laminectomy with placement of a shunt (Fig. 4–4). The patient underwent placement of a tracheostomy, followed by an EATO decompression of his posteriorly migrated dens and caudal clivus and by an occipitocervical fusion performed under a separate anesthesia (Fig. 4–5). He was decannulated at 3 weeks postoperatively and had complete resolution of his erectile dysfunction as well as an improvement in his swallowing function with removal of his G-tube. By his 6-month follow-up visit, he was ambulatory with a quad cane. The endoscopic-assisted transoral approach presents a viable alternative to the standard microsurgical transoral approach for treating anterior compressive lesions at the craniovertebral junction. The ability with endoscopic-assisted surgery to look superoinferiorly, laterally, and around the corners often obviates the need for extensive soft palate splitting, hard palate resection, or extended exposures. REFERENCES
4

Endoscopic-Assisted Transoral Odontoidectomy

< div class='tao-gold-member'>
Endoscopic-Assisted Transoral Odontoidectomy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








