Ependymomas
Ependymoma is the third most common pediatric brain tumor, following medulloblastoma and astrocytoma in incidence. Pediatric neurosurgeons play an important role in treatment because ependyhmoma with gross total resection (GTR) have a high likelihood of cure, whereas those who do not fare poorly. Current standard therapy consists of GTR if possible, followed by local radiotherapy. Chemotherapy plays a less well-defined role in a minority of patients. Although many children with ependymoma are cured, a minority of children still die of this disease, illustrating the need for further clinical and basic science research on ependymomas.
41.1 Epidemiology, Clinical Presentation, and Pathogenesis
Neurosurgeons often speak of ependymomas as a homogeneous group of tumors despite several histologic variants, each with a distinct biology. Even among those tumors that are diagnosed as classic ependymomas on histopathology, there are several different types, again each with its own biology.1,2 Most current clinical and basic science studies deal with the ependymomas as a group, even though they diverge in regard to epidemiology, natural history, pathophysiology, and response to treatment.
The mean age at the diagnosis of pediatric ependymoma is 4 to 6 years, with about one-third of tumors diagnosed before the age of 3 years.3–7 Approximately 10% of pediatric brain tumors belong to the ependymoma group of tumors, comprising a much higher percentage than in the brain tumors of adults. Historical 5-year survival estimates range from 50 to 64%, with the progression-free survival (PFS) estimated rate lower, at 23 to 45%.6,8–11 In most cases, recurrence is found at the site of the original tumor, with isolated metastatic recurrence occurring in fewer than 20% of cases. Most children who have no evidence of disease at 5 years will be cured. The median time to recurrence is 13 to 25 months.32,69,74,76,83 Strikingly different from patients with medulloblastoma, those with ependymomas present with evidence of leptomeningeal dissemination at the time of diagnosis in only 5% of cases.6 The diagnosis of leptomeningeal metastases is made by contrast magnetic resonance (MR) imaging and/or cytologic examination of the cerebrospinal fluid (CSF). The results of both must be negative for a child to be ruled without metastatic disease. In children with ependymoma and a ventriculoperitoneal (VP) shunt, CSF obtained by lumbar puncture is more sensitive in detecting leptomeningeal disease.12
Relatively little is known of the pathogenesis of ependymomas compared with other childhood brain tumors. Ependymomas are thought to arise from radial glial cell precursors.13 In childhood, 90% of ependymomas are located intracranially, one-third supratentorially, and two-thirds infratentorially (▶ Fig. 41.1).14 Sixty percent of supratentorial ependymomas are in or adjacent to the ventricles, whereas the other 40% arise away from ependymal surfaces (▶ Fig. 41.2).
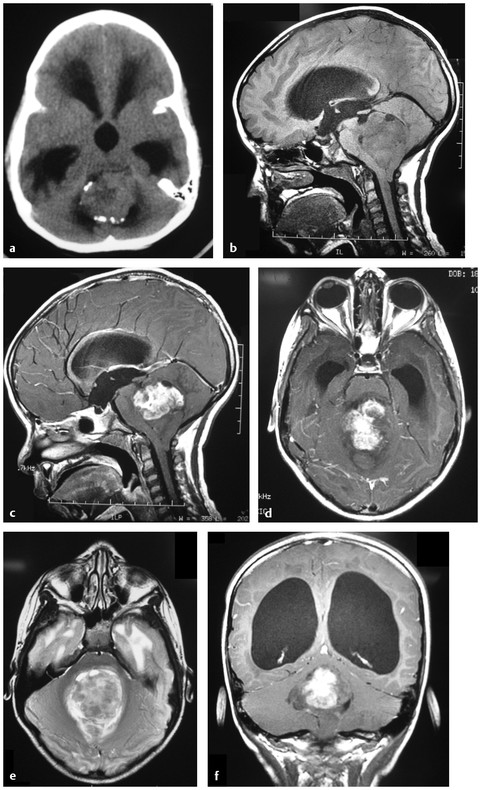
Fig. 41.1 a A 5-year-old with headaches and vomiting. (a) An unenhanced computed tomography (CT) scan shows a posterior fossa tumor, with some peripheral calcifications. There is associated supratentorial noncommunicating hydrocephalus from obstruction of the fourth ventricle. (b) Sagittal T1-weighted magnetic resonance (MR) image without intravenous contrast shows a mass lesion in the fourth ventricle that is isointense or hypointense to the brain. Note that the tumor and the cerebellum have protruded down through the foramen magnum. (c) Sagittal T1-weighted MR image with intravenous contrast shows enhancement of the superior portion of the tumor. One of the critical pitfalls in ependymoma surgery is failure to recognize the nonenhancing portion of the tumor. (d) Axial T1-weighted MR image with contrast. (e) Axial T2-weighted MR image shows a high-signal mass lesion in the fourth ventricle. (f) Coronal T1-weighted MR image with contrast shows supratentorial hydrocephalus and the partially enhancing, partially nonenhancing tumor. The nonenhancing portion of the tumor is extending out through the foramen of Magendie.
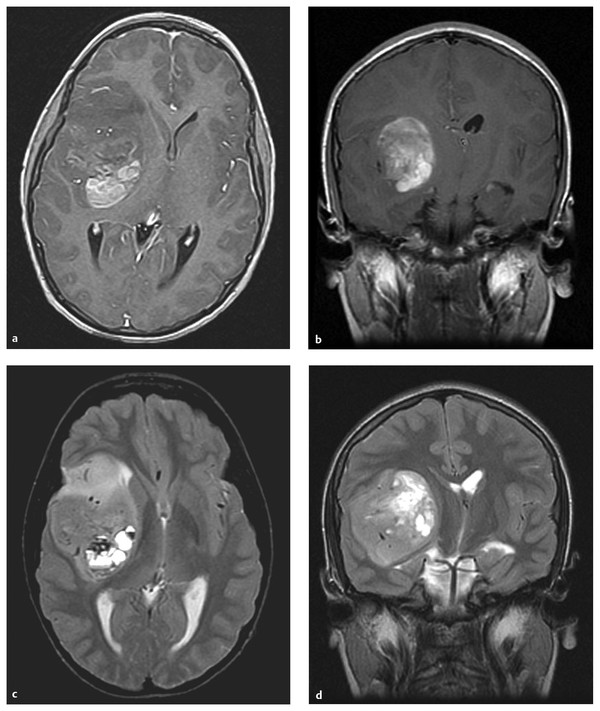
Fig. 41.2 Supratentorial ependymoma. (a) Axial T1-weighted magnetic resonance (MR) image with gadolinium shows a hemispheric mass lesion, partially enhancing, partially nonenhancing, in the right frontal operculum. (b) Coronal T1-weighted MR image with gadolinium. (c) Axial T2-weighted MR image. Note the paucity of peritumoral edema. (d) Coronal T2-weighted MR image.
Infratentorial (posterior fossa) ependymomas are thought to arise from three different sites in the fourth ventricle: the floor (60%), the lateral aspect (30%), and the roof (10%).15,16 Tumors that arise from the floor often extend out through the foramen of Magendie over the dorsal aspect of the spinal cord. Tumors arising more laterally may extend out of the foramen of Luschka to involve the contents of the cerebellopontine angle (CPA).
41.2 Outcome and Prognostic Factors
Many authors have examined prognostic factors in ependymoma. The most important prognostic factor is extent of resection. Other clinical variables thought to be prognostic include extent of disease at diagnosis (M status), tumor location, tumor grade, and patient age at diagnosis. There is no formally recognized staging system for ependymomas, although the importance of leptomeningeal dissemination is well known. Children are staged at the time of diagnosis or shortly after surgery with cranial and spinal MR imaging as well as examination of cerebrospinal fluid (CSF) cytology. Long-term survival is uncommon in children with M+ disease.
41.2.1 Extent of Resection
The single most important determinant of outcome in cases of pediatric ependymoma is the extent of surgical resection. The pediatric neurosurgeon must achieve a maximal safe resection. The 5-year survival rate in children who receive a GTR is 67 to 80%, and the 5-year PFS rate is 51 to 75%.4–7,9–11,17–25 In striking comparison, the 5-year survival rate of children who undergo subtotal resection is 22 to 47%, and 5-year PFS rate is 0 to 26%. Thus, many authors suggest that pediatric patients with ependymoma and residual tumor on postoperative imaging undergo a repeated resection.16,19,26,27
Many neurosurgeons classify ependymoma resections as gross total resection (GTR), near-total resection (NTR), subtotal resection (STR), and biopsy. The extent of surgical resection should be confirmed by postoperative MR imaging with and without gadolinium within 72 hours of resection to avoid confusing postoperative artifacts. A GTR is defined as absence of tumor on postoperative MR imaging. If a thin carpet of tumor is left on the floor of the fourth ventricle (and is visualized with the operating microscope at the time of surgery), but there is no evidence of tumor on postoperative MR imaging, this still qualifies as a GTR at St. Jude Children’s Research Hospital (Memphis, Tennessee). A resection is subtotal when gross residual tumor (more than a fourth ventricular layer) remains after surgery that was visible to the surgeon or evident on neuroimaging. Although NTR was once thought to be prognostically similar to GTR and has been arbitrarily defined as less than 1.5 cm3 on postoperative imaging (similar to the values quoted for medulloblastoma) and/or a 0.5-cm residual thickness of tumor bed enhancement, longer follow-up has shown that these patients actually stratify more closely to patients with STR, making us even more aggressive about a return to surgery for small amounts of residual tumor; however, we do not return to surgery for a layer of tumor invading the floor of the fourth ventricle unless it is bulky disease.11,19 Recent studies, based upon molecular profiling of ependymomas, suggest that fourth ventricular ependymomas that invade the floor of the fourth ventricle have a better prognosis than do lateral ependymomas, regardless of the extent of resection.2 This is a very important concept for the pediatric neurosurgeon; it is not necessary to perform extensive, overly aggressive resections in this location (i.e., chase an ependymoma into the floor of the fourth ventricle) because doing so will not increase the chance of cure enough to justify the risk for harm. The most valuable tool in the assessment of extent of resection is postoperative MR imaging because the surgeon’s judgment is notoriously suspect.
41.2.2 Histologic Grading
There are numerous articles in the literature both supporting and refuting the value of histologic grading in determining the prognosis of patients with ependymoma.6,11,20,22,23,28–31 Anaplastic ependymoma exhibits histologic evidence of advanced anaplasia, including nuclear atypia, marked mitotic activity, a high level of cellularity, microvascular proliferation, and/or necrosis. Part of the reason for the controversy in the literature is the inability of pathologists to agree on what constitutes an anaplastic ependymoma. A study from the Children’s Cancer Group (CCG) showed that 22 of 32 cases (69%) had a discrepancy in the diagnosis at the time of central pathologic review.11 Ellison et al reported less than 50% agreement among five experienced neuropathologists across three multicenter studies of ependymoma. Four of five agreed only 70% of the time.32
Analysis of a group of 50 contemporary patients with ependymoma from St. Jude Children’s Research Hospital showed that tumor grade was significantly related to PFS after irradiation (p < 0.001).19 In this group of patients, 2-year event-free survival (EFS) was 32% ± 14% for children with anaplastic ependymoma and 84% ± 7% for children with classic ependymoma. This association remained significant after adjustment for ages less than 3 years, chemotherapy, and extent of resection. Supratentorial ependymomas were more likely than posterior fossa ependymomas to show anaplastic histology.
Recently, attempts at more simple risk stratification have been reported.33 Gain of chromosome 1q and STR reliably placed patients into a high-risk category independently of histopathologic analysis. Cell density and mitotic count were used in the model to add specificity; however, mitotic count did stratify out an intermediate-risk category (▶ Fig. 41.3). It did not improve the prediction over 1q status and totality of resection alone.
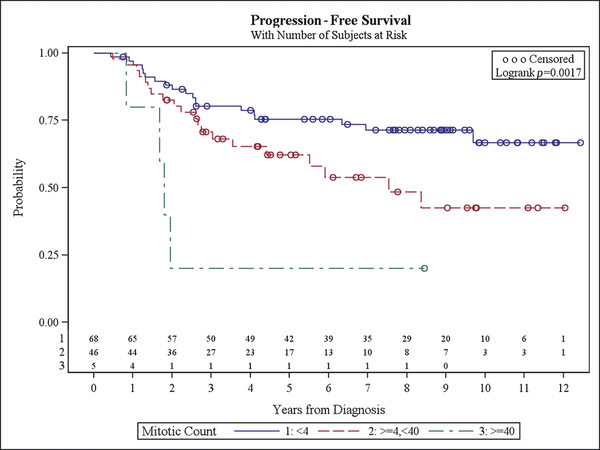
Fig. 41.3 Progression-free survival with number of subjects at risk.
(Courtesy of David Ellison.)
41.2.3 Age at Diagnosis
Children less than 3 years of age at the time of ependymoma diagnosis have a poor prognosis when compared with older children. Several factors may account for this. Tumors in children of this age have a different molecular pathophysiology (see below) and may have more aggressive clinical behavior. Younger children are more fragile and have increased complications of surgery, radiation, and chemotherapy. Clinicians are reluctant to give radiotherapy to children less than 3 years old, or they give lower doses of radiation supplemented by chemotherapy. Pollack reported a 5-year survival rate of 22% and a PFS rate of 12% in children less than 3 years of age. Older children had 5-year survival rates of 75%, and the PFS rate was 60%.10 A study by the Pediatric Oncology Group (POG) showed a 63% 5-year survival rate for children 24 to 35 months of age (with radiation delayed 1 year by the use of chemotherapy), but only a 26% 5-year survival rate for younger children (0 to 23 months, radiation delayed 2 years).34 The data of Merchant would suggest that the poorer prognosis in young children is more likely related to delayed or reduced-dose radiation. Historically, children who receive radiotherapy have had an increased survival compared with those who do not.35,36
41.2.4 Pathology
Ependymomas are classified as glial tumors, and their diagnosis is often straightforward. Histologic grading is more difficult, and it is controversial, as discussed earlier. A pathologic diagnosis is made by histologic examination supplemented by immunohistochemistry. Ependymomas are moderately cellular neoplasms with monomorphic nuclei. Perivascular pseudorosettes and less commonly true ependymal rosettes can be seen on histology. Endothelial proliferation is seldom seen in classic ependymoma. The World Health Organization (WHO) classification defines grade II ependymoma as follows: mitoses rare or absent; occasional foci of palisading necrosis; nodules with increased cellularity, and mitotic activity.37 Ependymomas usually stain positively for glial fibrillary acidic protein (GFAP).
Anaplastic ependymoma (AE) is a grade III lesion in the WHO classification.37 Tumors with clear ependymal differentiation, perivascular pseudorosettes, increased cellularity, cytologic atypia, and microvascular atypia are diagnosed as AE. Areas of hypercellularity may be diffuse or focal and may form well-circumscribed regions. Additionally, areas of cytologic atypia, including an increased nuclear-to-cytoplasmic ratio and cellular pleomorphism, may be seen. Anaplastic regions often have a higher mitotic rate, although no specific threshold for a diagnosis of AE is in wide acceptance. The 2000 WHO criteria for AE include increased cellularity, brisk mitotic activity, vascular proliferation, and pseudopalisading necrosis.38 Neither focal areas of atypia nor brisk mitotic activity is sufficient to make a diagnosis of AE. It is unclear whether AE arises from progression or malignant degeneration of classic ependymoma or if it occurs de novo. Failure to find true ependymal rosettes or perivascular pseudorosettes is associated with a poor prognosis in children with ependymoma.17 Two separate groups have reported that the combination of necrosis, endothelial proliferation, and a mitotic index above 5 was a negative predictive factor for overall survival (OS) and PFS.17,39
There is an inverse relationship between survival and mitotic rate demonstrated by proliferating cell nuclear antigen (PCNA), Ki-67, and MIB-1 labeling studies of ependymomas.17,25,39–41 However, one group found that mitotic rate was important only in determining the prognosis of supratentorial ependymomas.42
Worse survival is related to increased expression of the following: p53, topoisomerase II-α, B-cell lymphoma-2 (Bcl-2), tenascin, vascular endothelial growth factor (VEGF), and epidermal growth factor receptor (EGFR).25,43,44 Nuclear expression of the apoptotic protein Survivin portends a good prognosis in breast and gastric cancer, and low levels of nuclear Survivin were seen with higher-grade ependymoma than with classic ependymoma.45 Although these various molecular findings require further verification with prospective data, they may represent an additional opportunity to stratify ependymomas objectively, beyond the gain of chromosome 1q mentioned earlier, based on their biology.33
There is mounting evidence that among the classic supra- and infratentorial and spinal ependymomas there are distinct cytogenetic differences that dictate their biology. These differences are likely based on differing progenitor cells or differentiation at different points during embryologic development. The group at St. Jude Children’s Research Hospital has made significant advances in understanding the molecular basis of ependymomas. They used mRNA profiles to segregate ependymomas by central nervous system (CNS) location and unmasked previously unknown subgroups among supratentorial, posterior fossa, and spinal ependymomas, classifying ependymomas into nine distinct subgroups (subgroups A through I).1 They identified potential ependymoma oncogenes, which included several regulators of stem cell proliferation, pluripotency, and neural differentiation. These include THAP11, PSPH, EPHB2, 10 genes within the PCDH cluster, KCNN1, RAB3A, PTPRN2, and NOTCH1. This finding allowed the researchers to create the first ependymoma mouse model. The specific combination of embryonic cerebral radial glial cells, deletion of Ink4a/Arf, and amplification of EphB2 generated supratentorial ependymomas.1
Researchers from Toronto and Heidelberg confirmed these findings in 2011 with a study that examined two independent cohorts of patients with posterior fossa ependymoma.2 Using a combination of cytogenetic techniques, they grouped tumors into three distinct biological groups—a supratentorial ependymoma and two posterior fossa subtypes. The two posterior fossa subtypes were indistinguishable under the microscope. The groups were segregated into group A (younger, male, balanced genome, WHO grade III, CPA location, invasive, recurrent, metastatic, worse survival) and group B (older, gender-balanced, unbalanced genome, fourth ventricular location, rarely invasive, rarely recurrent, rarely metastatic, good survival). PFS and OS rates for group A were 44% and 65%, respectively. The corresponding rates in group B were 75% and 95%, indicating that although the tumors are histologically identical, they are distinctly different diseases. The investigators found group A tumors to be LAMA2+/NELL2- and group B tumors to be LAMA2-/NELL2+.
The ability to stratify ependymoma patients will allow clinicians to identify which of them will require more aggressive and/or novel therapies. Future clinical trials should include an assessment of the status of these markers and their correlation with response to therapy, PFS, and OS.
41.3 Types of Ependymoma
There are several variants of ependymoma that have distinct histologic appearances or that exhibit unique biology. These ependymoma variants all show evidence of ependymal differentiation on electron microscopy; however, they likely represent entities that are distinct and separate from classic ependymoma. Thus, a collection of diseases exists that should be called the ependymomas.
41.3.1 Myxopapillary Ependymomas
This variant of ependymoma occurs in the filum terminale or conus (▶ Fig. 41.4) and in the adult literature is very indolent. In the pediatric literature, myxopapillary ependymomas are not so indolent; they are more likely to recur and to metastasize.46,47 Patients typically present with pain, lower extremity weakness, numbness, and bladder dysfunction.48 The diagnosis is often delayed because of the nonspecific nature of the presentation.49 Myxopapillary ependymoma appears on imaging as an extramedullary tumor, or as a tumor growing out of the conus (▶ Fig. 41.4). Compared with other spinal tumors, myxopapillary ependymomas can have a high signal on T1-weighted MR imaging, perhaps because of their high content of mucin.50 Myxopapillary ependymomas in adults are cured with total excision. Less so in children. Subtotal resection followed by local radiation therapy is used to minimize neurologic deficits in cases in which the tumor is deemed unresectable.48,51,52 Uncommonly, myxopapillary ependymomas may metastasize, at which time they can be successfully treated with craniospinal irradiation, with the recognition that craniospinal irradiation in children is quite morbid.53 As such, the authors feel strongly that incompletely resected myxopapillary ependymomas in children should not be observed but should be treated, either with return to surgery for GTR or with focal irradiation if unresectable. Histologically, the tumors appear as cuboidal cells arranged around a fibrovascular core with extensive areas of intrapapillary mucin. Myxopapillary ependymomas show a distinct biology; gains of chromosomal material on chromosomes 9 and 18 are seen in most tumors that are not commonly seen in classic ependymoma. It remains unclear what biological relationship (if any) myxopapillary ependymomas have to classic ependymomas.
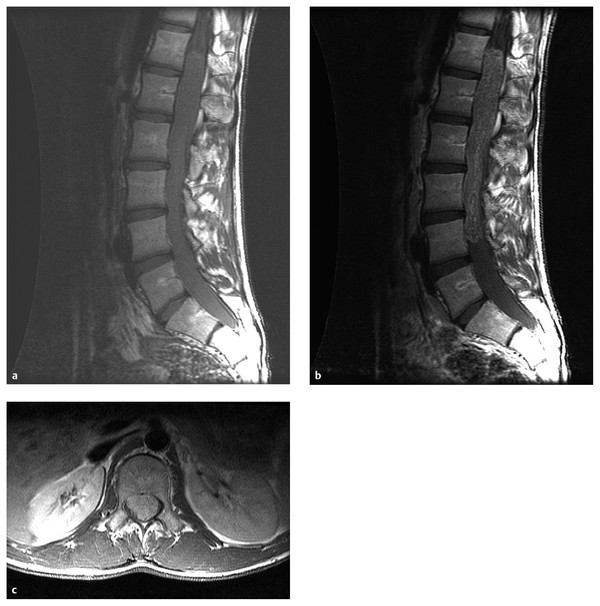
Fig. 41.4 Myxopapillary ependymoma in a child with back pain. (a) Sagittal T1-weighted magnetic resonance (MR) image without contrast shows an intradural tumor filling the thecal sac in the low thoracic and lumbar spine. (b) Added intravenous contrast better defines the tumor and shows that it extends from T12 to L4. (c) Axial image shows the tumor filling the thecal sac, displacing the nerve roots to the periphery of the sac.
41.3.2 Clear Cell Ependymomas
This ependymoma variant occurs preferentially in children and is usually found in the supratentorial compartment.54 Histologically, it may be mistaken for other clear cell neoplasms, including oligodendroglioma, central neurocytoma, hemangioblastoma, and renal cell carcinoma.54 Electron microscopy is invaluable in the diagnosis of this variant; it shows ependymal features, including microvilli, cilia, and junctional complexes.55,56 Cysts with enhancing walls were seen in 9 of 10 patients with clear cell ependymoma.54 The PFS and OS rates at 5 years in a recent series of clear cell ependymomas were 34% ± 20% and 75% ± 19%, worse than those for classic ependymoma.54 In that series, 2 of 10 patients developed extra-CNS metastases.54 Clear cell ependymomas showed frequent loss of genetic material on chromosome 18, a finding specific to the clear cell variant that is not commonly seen in classic ependymoma. These are probably biologically unrelated tumors.
41.3.3 Intramedullary Spinal Cord Ependymomas
Ependymomas within the spinal cord (▶ Fig. 41.5) typically present with myelopathy, back pain, weakness, bowel and/or bladder deficit, or scoliosis.57 The presence of an intramedullary ependymoma of the spinal cord in a young child should prompt one to consider neurofibromatosis type 2 (NF-2).
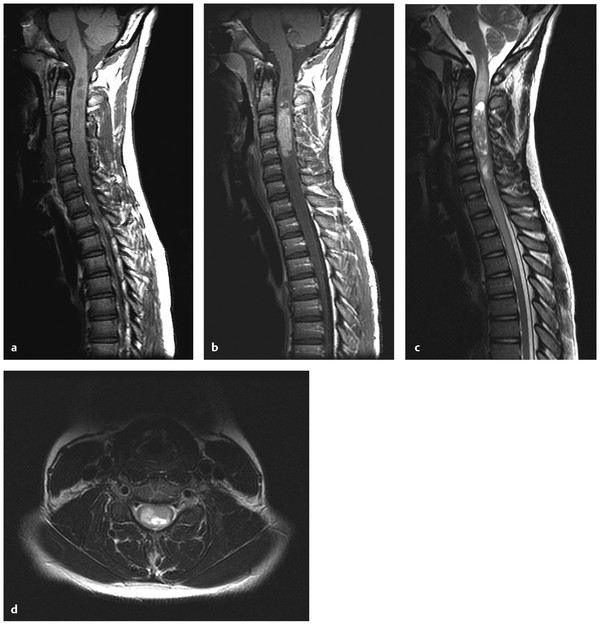
Fig. 41.5 (a) Cervical intramedullary ependymoma. (a) Sagittal T1-weighted magnetic resonance (MR) image without contrast shows areas of low signal within an expanded cervical spinal cord. (b) Sagittal T1-weighted MR image with contrast shows an enhancing mass lesion (ependymoma) within the cervical spinal cord. Cystic areas (syrinxes) are seen both superior and inferior to the mass lesion. (c) Sagittal T2-weighted MR image shows the intramedullary tumor and the adjacent syrinxes. Edema of the spinal cord is seen extending rostrally to the brain stem. (d) Axial T2-weighted MR image shows expansion of the cord with abnormal signal.
Intramedullary ependymomas are more discrete than intramedullary fibrillary astrocytomas and thus are easier to completely resect because of the presence of an obvious tumor–spinal cord interface. Current treatment for these rare tumors at St. Jude is maximal safe resection followed by focal radiotherapy.58 Of only 4 patients with intramedullary ependymoma treated at St. Jude, 2 are dead of disease.58 In a recent mixed series of 67 adult and pediatric tumors, GTR was the stated preoperative goal, and when it was achieved (55 cases), no postoperative radiation was performed.57 There were only 3 recurrences in the patients with initial GTR, and 2 underwent repeated operation for GTR. Radiation was reserved for patients in whom GTR was not achieved. Although intramedullary ependymomas are histologically similar to intracranial ependymomas, they have a distinct biology. Patients with NF-2 are prone to developing spinal ependymomas. Moreover, sporadic intramedullary ependymomas, but not sporadic intracranial ependymomas, have somatic mutations of the NF2 gene. This demonstrates that although intracranial and intraspinal ependymomas are histologically similar, they differ in biology and thus would be expected to have distinct natural histories and responses to therapy.
41.3.4 Tanycytic Ependymomas
Tanycytic ependymomas are an uncommon variant, with fibrillary cells that usually extend to the lumen of the ventricle.59 They may occur within the brain or the spinal cord.59,60 True ependymal rosettes are absent, and perivascular pseudorosettes are sparse.59 This ependymoma variant is often difficult to differentiate from an astrocytoma or a schwannoma. Immunohistochemistry is positive for GFAP and S-100.59 This variant is thought to arise from tanycytes, a group of elongated unipolar and bipolar cells that extend between the ventricular lumen and the surface of the CNS. The ependymal nature of these tumors has been demonstrated based on their electron microscopy characteristics.59 Although reports of tanycytic ependymoma are sparse, it should be recognized. It is likely an indolent variant, and aggressive therapies should be used with caution.59,60
41.4 Genetics
Intramedullary spinal ependymoma is well known to occur in patients with NF-2; however, patients with NF-2 are not at increased risk for the development of intracranial ependymomas. Mutations of the NF2 gene, located on chromosome 22q, are not found in intracranial ependymomas, myxopapillary ependymomas, or tanycytic ependymomas.61,62 Although intracranial ependymomas are well known to show loss of genetic material on 22q, they do not harbor NF2 mutations, suggesting the existence of another, distinct ependymoma tumor suppressor gene in this region of the genome.
Classic ependymoma is reported in patients with the Li–Fraumeni familial cancer syndrome (TP53 tumor suppressor gene).63,64 However, somatic mutations of TP53 are not commonly found in sporadic ependymomas and likely do not play a large role in their pathogenesis.65,66 Scattered reports of patients with ependymoma and Turcot syndrome (brain tumors plus colonic neoplasia) are found in the literature, with some patients developing more than one ependymoma.67–69 These patients have germline mutations in the adenomatous polyposis coli (APC) gene on chromosome 5 and have overactivity of the Wnt signaling pathway. The role of the APC gene and/or Wnt signaling in sporadic ependymomas has not been elucidated. Spinal ependymomas have also been reported in the context of the multiple endocrine neoplasia type 1 syndrome due to mutation of a tumor suppressor gene on chromosome 11q13.70,71 Other families have an increased incidence of ependymomas, but no currently recognized familial tumor syndrome has been reported.72–74
41.4.1 Cytogenetics
As a group, ependymomas studied by G-banding karyotype show a frequent loss of genetic material on chromosomes 22q, 6q, 9q, 17p, and 11q and show a gain of genetic material on chromosome 1q.62,64,75–80 Loss of heterozygosity on chromosome 22q is more common in adult ependymomas and intramedullary spinal ependymomas and less common in pediatric ependymomas.64 There is some evidence that the gain of genetic material on chromosome 1q may be an early event in the initiation of ependymoma.75 A 1q gain stratifies patients into a higher-risk group.33 Subsequently, several groups have published studies in which the technique of comparative genomic hybridization was used to study ependymomas.81–87 This technique is more sensitive than a G-banding type karyotype at finding gains and losses of genetic material. Ependymomas from very young children frequently show a balanced karyotype (no observed gains or losses).81 Classic ependymomas from the brain and those from the spine have different cytogenetic profiles. Tumors with gain of chromosome 1q tend to occur in the posterior fossa in children, and the tumors often behave aggressively.81 Recurrent tumors show more extensive karyotypic abnormalities than the original tumors; whether this is due to biological progression or to random DNA damage from radiation and/or chemotherapy is not clear.82 Myxopapillary ependymomas show a higher number of cytogenetic abnormalities (average of nine per tumor) than do other ependymomas.85 The most common changes seen in cases of classic ependymoma are gains of chromosomal material on chromosome arm 1q or chromosome 9 and losses of 6q, 22, and the X chromosome.84
41.5 Imaging Studies
MR imaging is central to the diagnosis, management, and follow-up of children with ependymoma. Anatomical definition is required before surgery to determine the extent of tumor growth because some ependymomas have a propensity to grow out of the foramen of Luschka into the CPA or out of the foramen of Magendie into the cervical spine. MR imaging is also used to plan radiotherapy and to judge response to chemotherapy when it is given. Rarely, a child may present in extremis, and an operation will be done without preoperative MR imaging.
Most children with ependymoma should have pre- and postoperative imaging of the brain, and either pre- or postoperative imaging of the entire spine. Postoperative brain imaging should be done within 72 hours of surgery because after this time postsurgical artifact may make it difficult for radiologists to determine the extent of resection. If this 3-day window is missed, MR imaging should be delayed for a couple of weeks, at which time the artifact from surgery is decreased. Gelfoam (Pfizer, Cambridge, MA and La Jolla, CA) or Surgicel (Johnson & Johnson, New Brunswick, NJ), used for hemostasis at the time of surgery, can make the postoperative images difficult to interpret and may enhance over time, causing confusion with recurrent tumor. Leaving such material in the wound is therefore strongly discouraged.88
Most ependymomas are variably enhancing. The T2 image helps determine the true extent of the tumor. Pre- and postoperative T1 films with and without gadolinium enhancement, as well as T2-weighted imaging, are necessary for comparison. Both the location and the extent of residual tumor are important in formulating a therapeutic plan.
The optimal frequency of surveillance imaging after treatment of ependymoma is uncertain. Most children with recurrence or progression present between 12 and 24 months after the initiation of radiation therapy. Early detection of recurrence should maximize the opportunity for salvage therapies. Some retrospective trials suggest that the early detection of asymptomatic recurrence of ependymoma leads to better outcomes than diagnosis when the recurrence becomes symptomatic.89,90
Preoperative spinal imaging identifies patients (approximately 5%) who present with leptomeningeal dissemination. Subsequent surveillance imaging of the spine is problematic because there are many mimics of leptomeningeal disease, including blood products, infection, and inflammatory changes secondary to surgery or radiation therapy. Any spinal imaging that cannot be performed before surgery should be performed at least 7 to 10 days after any invasive CNS procedure. Leptomeningeal tumor usually appears as a series of small nodular tumors, or as thick disease coating the subarachnoid space focally. Diffuse “sugar coating,” such as that seen in medulloblastoma, is seldom seen in ependymoma, and leptomeningeal should not be diagnosed if there have been any recent invasive procedures on the CNS.
Complete responses to either radiation or chemotherapy are uncommon in children with ependymoma, although partial responses are often seen. After radiation, most ependymomas will diminish in size over a period of years and many show loss of contrast enhancement due to a loss of vascularity. Conversely, some ependymomas may show an increase in enhancement for the first several months after radiation because of an increase in leukocyte–endothelial interactions. This should not be misinterpreted as progression of the tumor. Recurrences in the first year after adequate radiation therapy are uncommon. Similarly, responses to chemotherapy manifest approximately 6 months after the start of treatment.
41.6 Surgical Treatment
Most children with ependymoma present with localized disease. Most recurrences are local. Subarachnoid dissemination is rare and fatal. This disease is well suited to therapies designed to achieve local control. The overriding value of GTR of ependymoma has been demonstrated in several institutional retrospective reviews and two prospective Phase III trials.4–7,9–11,20,23,34 Ependymoma is a surgeon’s tumor because this portion of the patient’s care has the highest impact on the quality and quantity of life.
Sutton et al retrospectively analyzed 45 patients with ependymoma and found that the 5-year survival for GTR or NTR was 60%, but with STR (defined here as < 90% resection), it fell to 21%.7 Pollack et al found a 5-year survival rate of 80% after GTR, compared with 22% after less than GTR.10 Sutton et al retrospectively evaluated 92 children with ependymoma; the 10-year survival after GTR was 70%, and the PFS estimate was 57%.6 With STR, the 10-year survival was 32% and the 10-year PFS was 11%. Robertson et al prospectively treated 32 patients with CCG protocol 921 and found that the 5-year PFS rates were 66% for patients with less than 1.5 cm2 of residual tumor and 11% for those with more tumor.11 There is overwhelming evidence that cytoreductive surgery is beneficial to children with ependymoma and gives the best chance for long-term survival.
Two groups reported surgery without adjuvant treatments (i.e., radiation or chemotherapy) for children with ependymoma.21,88 Hukin et al described 10 pediatric patients who received only surgery as treatment for ependymoma; 8 tumors were supratentorial and 2 were in the posterior fossa.88 At a median of 48 months of follow-up, 7 of the 10 patients were free of disease without having received adjuvant therapy. The other 3 patients had documented tumor recurrence at 9, 10, and 20 months after resection. Of the 3 patients, 2 were effectively treated with repeated surgery and radiation. Of the other 7 patients, another 2 went on to late failures. Little et al argued that deferring radiation in low-risk patients (older than 3 years, GTR, focal disease, lack of high-grade features) was a reasonable option.91 Palma et al reported success in treating supratentorial ependymomas with surgery alone.21 Surgery alone is probably a reasonable therapeutic option for children with supratentorial ependymoma with low-grade histology when the surgeon is able to take a rim of white matter around the tumor.
Although complete resection is of paramount importance in the treatment of ependymoma, it is achieved in only 42 to 70% of patients at initial resection.5,7,10,23,92 Complete resection is more readily achieved in supratentorial tumors and those that arise from the roof of the fourth ventricle. GTR of tumors that invade the floor of the fourth ventricle or pass out the foramen of Luschka to involve the lower cranial nerves is much more difficult, and surgical complication rates are higher.
41.6.1 Treatment of Residual or Recurrent Ependymoma
Most children who initially receive less than an NTR should have repeated surgery to reduce their status to minimal residual disease (GTR or NTR) (▶ Fig. 41.6). The exception to this may be those rare cases in which resection of the residual tumor will result in intolerable morbidity. In the case of surgery that is abandoned because of excessive bleeding, chemotherapy between resections may decrease the vascularity of the tumor, thus facilitating a safe return for repeated resection.93 An evaluation of the initial approach may make obvious why the initial resection was STR. An alternate approach may make previously “hidden” tumor easy to resect. Second resections for ependymoma have an acceptable morbidity.26 Although some complications were seen in 45% of children undergoing second-look surgery for pediatric CNS tumors, there was no significant change in mean functional scale scores at 4 or 24 weeks after surgery.94 Foreman et al achieved GTR in four-fifths of a group of patients with ependymoma at the time of second surgery with no severe morbidity, and three of the patients remained progression-free at 23, 25, and 34 months after radiation therapy.26
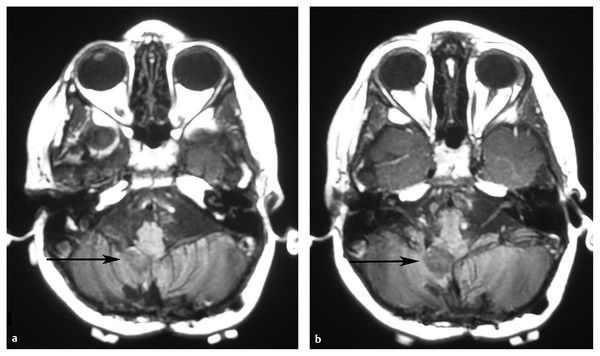
Fig. 41.6 Residual ependymoma after a first resection. (a,b) Postoperative magnetic resonance images after resection of a pediatric posterior fossa ependymoma show a small amount of nonenhancing residual tumor in the right foramen of Luschka. This residual tumor was resected at a repeated craniotomy, yielding a gross total resection.









