Epilepsies Characterized by Partial Seizures
Focal seizure is the term proposed by the Task Force of the International League Against Epilepsy (ILAE) to designate seizures in which the first clinical symptoms indicate the paroxysmal activation of an anatomofunctional system of neurons limited to part of a single hemisphere, with a correspondingly localized electroencephalographic (EEG) discharge (Engel, 2001). The terms localization-related epileptic seizures or focal seizures designate the same type of seizures. Focal seizures are often associated with generalized seizures in the same patient. In such patients, the epilepsy is usually considered to be focal because, in these patients, generalization is considered to be secondary to an initial focal seizure of partial seizures. Epilepsies with partial seizures are the most common form of epilepsy (Zarrelli et al., 1999).
Epilepsies with focal seizures were divided into the following two groups by the ILAE seizure classification of 1989: those with elementary (simple partial seizures) and those with complex (complex partial seizures) symptomatology (Commission on Classification and Terminology of the International League Against Epilepsy, 1981). Impairment of consciousness is defined operationally by the responsiveness and awareness of the patient during the seizure. It is preserved in simple partial seizures and is decreased or abolished in complex focal seizures, and it was the criterion for separating the two types. This criterion has been disputed, and the proposal for a new classification (Engel, 2001) does not include these terms.
This chapter describes the symptomatology of focal seizures without specific referencing to the dichotomy previously used. It is divided into three parts. The first part gives a description of the multiple types of focal seizures according to the nature of their clinical ictal manifestations; it successively considers motor, sensory, autonomic, and cognitive symptoms and impairment of consciousness, with or without associated cognitive or affective features. This section describes the topographic grouping of seizure symptoms according to the cortical areas involved in the discharges. The reader should keep in mind that the areas involved by the first clinical and/or EEG manifestations are not necessarily those in which the discharge actually originates; they can be secondarily involved by its propagation. The second part is concerned with the description of the various recognizable topographic epilepsy syndromes that feature focal seizures with or without impairment of consciousness. The third part is concerned with the syndromes that are predominantly manifested by focal seizures.
SEIZURE SYMPTOMATOLOGY
FOCAL MOTOR SEIZURES
Motor seizures are characterized by motor symptoms as their main clinical manifestation. The ictal symptomatology may be either simple (a jerk) or complex (complex organized movements). Focal clonic and myoclonic muscle activity typically arises from seizure discharges in the primary motor cortex. Tonic muscular activity is typically produced by the activation of the premotor areas (Rasmussen, 1974) or the supplementary sensorimotor cortex (Penfield and Jasper, 1954). The epileptic discharges responsible for partial motor seizures may remain localized to a relatively small cortical area, which, in childhood, is often the lowermost part of the motor strip, but they may also spread slowly from their area of origin to involve the neighboring areas. When spread occurs within the motor or sensory strips, a progressive and regular extension of the territory involved in the convulsive activity or the paresthesias results. This jacksonian march often stops before affecting the whole motor or sensory area. A typical jacksonian march is uncommon. More commonly, the extension of a localized discharge is irregular. For example, a discharge that starts in the primary motor cortex may involve subcortical structures that, in turn, activate other cortical areas that may be relatively distant from the site of origin (Fig. 10.1). This type of propagation explains the common saltatory expression of many simple partial seizures, with their sequences of jacksonian march alternating with “jumps” of the paroxysmal activity to other parts of the body. Although distinctive patterns of motor activation of the primary motor cortex, premotor areas, and supplementary
sensorimotor cortex may be observed, overlapping clinical manifestations also exist due to highly developed interconnectivity of the different cortical motor areas.
sensorimotor cortex may be observed, overlapping clinical manifestations also exist due to highly developed interconnectivity of the different cortical motor areas.
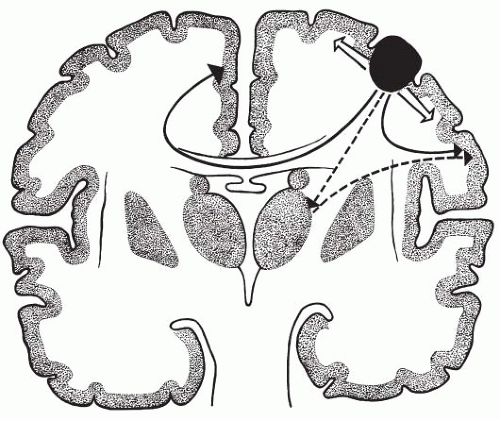 FIG. 10.1. Schematic concept of the various types of propagation of partial motor seizures. From its area of origin (epileptogenic zone) in the motor strip, the discharge may spread slowly along the motor strip (open arrows), but it may also move rapidly through normal anatomic pathways or through abnormally facilitated pathways to distant areas on the same or opposite side (solid arrows). This long-distance propagation explains the frequently irregular “saltatory” course of partial motor seizures and the occurrence of contralateral symptoms, particularly, ipsiversion of the head and eyes. A third unproved pathway (dotted arrows) may exist with activation of the corresponding area in the thalamus, which, in turn, would activate other cortical areas. (Adapted from Bancaud and Talairach, 1975.) |
Ictal Manifestations
Somatomotor Seizures with Simple Clonic and Tonic Phenomena
Somatomotor seizures with simple clonic and tonic phenomena are characterized by localized convulsive movements that are often clonic from the start or that become clonic after an initial brief tonic contraction. Clonic seizure activity is characterized by rhythmic jerking or twitching of usually contiguous body segments due to short (50-ms to 200-ms) muscle contractions that alternate with postjerk silent periods.
However, purely tonic focal motor seizures do occur (O’Neil et al., 1991). Any part of one side of the body can be affected. Because of the respective sizes of the cortical representation of movement, the seizures tend to involve the thumb, fingers, lips, eyelids, and great toe preferentially. In children, the involvement of the muscles of the face, tongue, pharynx, and larynx, with consequent salivation and dysarthria, is especially common. Facial buccolingual tonic-clonic contractures with aphemia point to the involvement of the lowermost part of the motor strip in the upper bank of the sylvian fissure (Loiseau and Beaussart, 1973; Lombroso, 1967). In some seizures, the convulsions remain narrowly localized (e.g., to one segment or limb). In jacksonian attacks, the symptoms travel slowly from one territory to another, following the order of their somatotopic representation, usually from the extremity to a proximal part. A jacksonian march or very narrowly localized clonic or tonic motor phenomenon is evidence favoring the primary involvement of the rolandic strip. Jacksonian seizures represent the only ictal symptom, thus allowing a seizure type to be assigned to a precise cortical area on the strength of clinical observation only (Chauvel et al., 1992b).
Partial motor seizures may vary from a few seconds to several hours in duration. When they are prolonged, they are usually clonic. Postictal hemiplegia, or a more restricted motor deficit (Todd paralysis), is common following long seizures, and it may last from minutes to several days. It may also be observed after brief seizures or even partial seizures without motor manifestations (Mauguière and Courjon, 1978; Gastaut and Broughton, 1972). The presence of Todd palsy indicates the focal character and location of the causal seizure when a precise history is lacking. When Todd paralysis lasts only minutes, it is probably caused by postictal inhibition. When it is more prolonged, it probably reflects the metabolic exhaustion of the cortical area involved in seizure activity or the existence of transient changes in local blood flow and/or the blood-brain barrier. Localized edema has been demonstrated in such cases (Sammaritano et al., 1985; Dillon et al., 1984).
Clonic seizures are often preceded by somatosensory auras (Mauguière and Courjon, 1978) that, however, are rarely reported in smaller children. Of 14 patients with clonic seizures, 7 were reported to harbor a structural lesion in the primary motor cortex (Geier et al., 1977). A total of 10 of 22 children with frontal lobe epilepsy studied with ictal single photon emission computed tomography (SPECT) by Harvey et al., (1993b) had clonic seizures that had been preceded by other symptoms, including a tonic phase in most. On the whole, clonic seizure onset appears to be more common with dorsolateral seizure onset than
with mesial frontal or orbitofrontal origin (Noachtar and Arnold, 2000). Clonic seizures, which were often preceded by a somatosensory aura, were observed in up to 57% of patients with parietal lobe seizure onset that was posterior to the postcentral sulcus (Salanova et al., 1995b). Clonic movements following tonic posturing also occur during seizures originating in the supplementary sensorimotor cortex (Morris et al., 1988). Clonic seizure activity, which is usually preceded by subjective symptoms and automatisms, has also been reported in about one-fourth to one-third of patients with temporal lobe seizure onset (Noachtar and Arnold, 2000).
with mesial frontal or orbitofrontal origin (Noachtar and Arnold, 2000). Clonic seizures, which were often preceded by a somatosensory aura, were observed in up to 57% of patients with parietal lobe seizure onset that was posterior to the postcentral sulcus (Salanova et al., 1995b). Clonic movements following tonic posturing also occur during seizures originating in the supplementary sensorimotor cortex (Morris et al., 1988). Clonic seizure activity, which is usually preceded by subjective symptoms and automatisms, has also been reported in about one-fourth to one-third of patients with temporal lobe seizure onset (Noachtar and Arnold, 2000).
Unilateral Clonic Seizures
Unilateral clonic seizures (Gastaut et al., 1974c), which are common in childhood, are characterized by synchronous rhythmic jerking of most or all of the muscles of one-half of the body. The distribution of the jerks can be stable or variable, migrating on the affected side (Dravet, 1992a). The jerks may sometimes be very mild, only affecting the eyeballs or the orbicularis oris; they may sometimes cease on one side, subsequently starting contralaterally. Unresponsiveness is common, but this is not always observed; it can be either initial or gradual. Seizure onset is usually represented by lateral clonic turning of the eyes or the head and eyes, often with vomiting. Autonomic phenomena, including pallor, perspiration, or hypersalivation, are common. The child may recover consciousness quickly as seizure activity ceases while postictal hemiparesis is still present (Dravet, 1992a). The severity and duration of postictal hemiparesis are related to the duration of the seizure. Very prolonged unilateral seizures may be followed by permanent hemiparesis (Guerrini and Dravet, 1997; Gastaut et al., 1974b).
Seizures with Asymmetric Tonic Motor Phenomena
Seizures with asymmetric tonic motor phenomena include differing manifestations. The term versive seizure was proposed by Gastaut and Broughton (1972) to designate ictal turning of the head and eyes. This is a common type of motor attack involving conjugate, unnatural deviation of the head and eyes to one side (Wyllie et al., 1986a, 1986b), with tonic or clonic components. The ictal version must be differentiated from head and eye turning that occurs in some partial seizures but is not due to direct involvement of the motor system by the discharge but to such factors as external stimuli, or a visual hallucination moving across his or her visual field, or ictal dysfunction of the attention mechanisms within the ipsilateral hemisphere (Chee, 2000). The deviation is most often away from the discharging hemisphere (contraversive seizures), but it may be toward the side of ictal EEG activity (ipsiversive seizures) (Ochs et al., 1984). Version is subserved by different reciprocally interconnected, discrete anatomic areas, including the frontal eye field, supplementary eye field, parietal eye field, calcarine cortex, and subcortical structures (Chee, 2000) that, when stimulated, produce similar clinical manifestations (Chee 2000; Fish et al., 1993). As a result, the localizing and lateralizing value of head and eye deviation is limited per se (Ochs et al., 1984; Robillard et al., 1983). However, a forceful tonic version that usually is accompanied by preservation of awareness seems to be preferentially associated with contralateral frontal origin in the dorsolateral cortex (area 8), and it may even be clonic (Jayakar et al., 1992; Wyllie et al., 1986a, 1986b). Clonic head version immediately preceding secondary generalization lateralizes the ictal onset to the hemisphere contralateral to the side of head turning (Wyllie et al., 1986a).
Conscious slow and saccadic deviation of the head and eyes that is followed by clonic eye movements is seen with purely occipital onset seizures (Williamson et al., 1992b; Bancaud et al., 1965) that can be either contralateral or, more rarely, ipsilateral (Bancaud, 1969). More complex attacks may comprise abrupt asymmetric tonic postural changes. These can be either a tonic flexion of the forearm, with abduction at the shoulder of the arm toward which the face is rotated and the assumption of an attitude reminiscent of the asymmetric tonic neck reflex, or, at times, repetitive vocalization. The lower limbs may also show abnormal posturing, with abduction at the hips and extension or semiflexion of the knees. This seizure pattern, which is also known as the M2e posture, has long been related to discharges involving the contralateral supplementary motor area on the internal aspect of the frontal lobe (Quesney et al., 1990; Ojemann and Ward, 1975; Ajmone-Marsan and Abraham, 1960; Ajmone-Marsan and Ralston, 1957), which, more recently, was defined as the supplementary sensorimotor area through electrical stimulation studies (Lim et al., 1994). Bilateral abduction of the upper limbs can also be observed (Morris et al., 1988). In some patients, extension of one upper limb with hyperextension of the arm and contralateral head rotation occur. Gyration of the whole body may be associated with head and eye deviation. Repetitive vocalization or loud moaning may be observed, and
some patients may try to speak during the attacks or may be able to respond as soon as the tonic contraction relaxes (Bleasel and Lüders, 2000; Remillard et al., 1974). However, speech arrest is more common. Because of the predominantly axial involvement and the preserved consciousness, the children may still demonstrate spontaneous activity, which, with modifications in the intensity and distribution of muscle contraction, may lead to slow writhing, dystonic movements (Bleasel and Lüders, 2000). Focal clonic activity indicates seizure spread to the primary motor cortex and makes lateralization obvious (Chauvel et al., 1992b). A sensation of tension or heaviness in a limb or a more general body or cephalic sensation is reported by some patients before the tonic contraction becomes apparent (Morris et al., 1988; Penfield and Jasper, 1954). The characteristics of such subjective symptoms are in keeping with the sensory representation of the supplementary sensorimotor area (Lim et al., 1994).
some patients may try to speak during the attacks or may be able to respond as soon as the tonic contraction relaxes (Bleasel and Lüders, 2000; Remillard et al., 1974). However, speech arrest is more common. Because of the predominantly axial involvement and the preserved consciousness, the children may still demonstrate spontaneous activity, which, with modifications in the intensity and distribution of muscle contraction, may lead to slow writhing, dystonic movements (Bleasel and Lüders, 2000). Focal clonic activity indicates seizure spread to the primary motor cortex and makes lateralization obvious (Chauvel et al., 1992b). A sensation of tension or heaviness in a limb or a more general body or cephalic sensation is reported by some patients before the tonic contraction becomes apparent (Morris et al., 1988; Penfield and Jasper, 1954). The characteristics of such subjective symptoms are in keeping with the sensory representation of the supplementary sensorimotor area (Lim et al., 1994).
Consciousness is most often preserved during attacks; the patient may, however, become unresponsive or a secondary generalization may follow. Supplementary motor seizures may be accompanied by unilateral tonic activity (Morris et al., 1988). The rapid spread of epileptic activity to the contralateral supplementary sensorimotor area is common (Baumgartner et al., 1996; Chauvel et al., 1992b; Morris et al., 1988), and this may account for some of the bilateral symptoms in some patients.
The seizure duration is brief, usually lasting between 10 and 40 seconds, and postictal confusion is rarely observed (Bleasel and Lüders, 2000). Childhood-onset seizures with bilateral asymmetric tonic activity and posturing are mainly seen in partial epilepsies of frontal lobe origin of either genetic or idiopathic or symptomatic origin (Scheffer et al., 1995a; Bleasel et al., 1993; Vigevano and Fusco, 1993).
Complex Motor Seizures with Hyperkinetic Automatisms: Complex Partial Seizures of Frontal Lobe Origin and Hypermotor Seizures
Some seizures feature prominent bilateral postural movements involving primarily the trunk, pelvis, and proximal extremities; they resemble natural movements and therefore differ from the tonic, clonic, or dystonic movements occurring during bilateral asymmetric motor phenomena. Numerous studies have tried to characterize such hyperkinetic postural phenomena and automatisms and their sequences. Different terms, such as extreme motor restlessness (Tharp, 1972), complex motor automatisms, agitation (Geier et al., 1977; Williamson et al., 1985; Manford et al., 1996a), or many others (Chauvel et al., 1995; Munari and Bancaud, 1992; Fusco et al., 1990; Delgado-Escueta et al., 1987; Waterman et al., 1987), have been used to define them. The resulting motor sequences may vary from seemingly natural movements with mild postural changes to large-amplitude, explosive movements involving different body segments. Even the more bizarre motor sequences tend to have a stereotypic pattern in the same patient (Lüders et al., 2000; Williamson et al., 1985). The characteristic patterns of repetitive movements and automatic activities include kicking, cycling, thrashing, crossing and uncrossing the legs, rocking, genital manipulations, a peculiar repetitive vocalization, echolalia, screaming, kneading of objects, crumbling, gripping something or somebody, rubbing, and rhythmic fine finger movements. Sometimes, movements suggest defensive or sexual behavior (Tinuper et al., 2001). Asymmetric tonic or dystonic limb posturing is common. Phonatory phenomena in the form of continuous or repetitive vocalization may be present (Bancaud et al., 1973). In children, aphemia without a disturbance in the understanding of language is more common than vocalization. Ictal autonomic changes, such as tachycardia or tachypnea, are common (Tinuper et al., 2001). Staring or a facial expression of surprise or fear is often noted. The degree of concomitant consciousness impairment varies greatly, ranging from full awareness to complete unresponsiveness (Holthausen and Hoppe, 2000; Chauvel et al., 1995; Williamson et al., 1985). The individual’s postictal recovery is rapid. Most studies have correlated these prominent motor manifestations with frontal lobe seizure activity involving the anterior cingulate and the orbitofrontal and frontopolar cortices (Chauvel et al., 1995; Bancaud and Talairach, 1992; Williamson et al., 1985).
Hypermotor seizures are characterized by “complex, organized movements that affect mainly the proximal portion of the limbs and lead to a marked increase in motor activity” (Lüders et al., 1993a, 1998). The term hypermotor is used in antithesis to hypomotor, which designates seizures characterized by a significant decrease in motor activity when ascertaining whether a concomitant alteration of consciousness (AC) has occurred is not possible (Lüders et al., 1998).
Purely “hypermotor” seizures occur in a minority of patients. In the great majority, the seizures include tonic phenomena that may be either mild or prominent (Holthausen and Hoppe, 2000). The order of the progression of motor manifestations is variable; the hypermotor behavior may precede or follow the tonic
movements (Holthausen and Hoppe, 2000; Chauvel et al., 1995; Geier et al., 1977). Subjective symptoms that precede the hypermotor seizures are reported in 50% to 90% of patients, and they mainly consist of a somatosensory aura in the form of abdominal aura or ictal fear (Biraben et al., 2001b; Tinuper et al., 2001; Holthausen and Hoppe, 2000; Harvey et al., 1993a; Williamson et al., 1985; Geier et al., 1977). “Hypermotor seizures” often have their onset in childhood (Provini et al., 1999; Vigevano and Fusco, 1993), with one series reporting such an onset in up to 90% (Holthausen and Hoppe, 2000). Onset in the first year of life has been reported with seizures characterized by an initial tonic type of posturing that occasionally is followed by hypermotor behavior (Vigevano and Fusco, 1993). When ictal fear is present, such episodes can be mistaken for pavor nocturnus (Chapter 21).
movements (Holthausen and Hoppe, 2000; Chauvel et al., 1995; Geier et al., 1977). Subjective symptoms that precede the hypermotor seizures are reported in 50% to 90% of patients, and they mainly consist of a somatosensory aura in the form of abdominal aura or ictal fear (Biraben et al., 2001b; Tinuper et al., 2001; Holthausen and Hoppe, 2000; Harvey et al., 1993a; Williamson et al., 1985; Geier et al., 1977). “Hypermotor seizures” often have their onset in childhood (Provini et al., 1999; Vigevano and Fusco, 1993), with one series reporting such an onset in up to 90% (Holthausen and Hoppe, 2000). Onset in the first year of life has been reported with seizures characterized by an initial tonic type of posturing that occasionally is followed by hypermotor behavior (Vigevano and Fusco, 1993). When ictal fear is present, such episodes can be mistaken for pavor nocturnus (Chapter 21).
The seizures tend to appear much more often during non-rapid eye movement (REM) sleep than during wakefulness (Tinuper et al., 2001; Holthausen and Hoppe, 2000; Fusco et al., 1990; Waterman et al., 1987). An arousal is often the first ictal manifestation during sleep (Fusco et al., 1990).
The motor activity usually lasts less than 1 minute (Provini et al., 1999; Tinuper et al., 2001), and, if no alteration of consciousness has occurred, the individual’s recovery is rapid. Secondary generalization is rare.
Paroxysmal Arousals and Epileptic Nocturnal Wandering
Paroxysmal arousals are short-lasting seizures (duration of 2 to 20 seconds) that occur during sleep and are characterized by a sudden brief arousal (Montagna et al., 1990). Opening of the eyes is often followed by sitting up in the bed with a frightened expression, with or without concomitant tonic or dystonic asymmetric posturing of the limbs.
Epileptic nocturnal wanderings (Plazzi et al., 1995; Pedley and Guilleminault, 1977) designate the presence of longer attacks (duration of 2 to 3 minutes) that appear during sleep and are characterized by an initial arousal, followed by semi-purposeful ambulatory behavior during which patients, who usually show a frightened expression and are particularly agitated, scream and may attempt to escape.
Aphemic Seizures
Aphemic seizures feature the incapacity to speak, to produce words, or to vocalize. They are related to speech arrest accompanying supplementary motor seizures, and they must be distinguished from ictal anarthria due to ictal activity in the pes of the third frontal gyrus and from pharyngeal constriction due to opercular involvement.
Focal Myoclonic Seizures
According to its distribution, myoclonus can be classified as focal, multifocal, or generalized (Hallet, 1985) (see also Chapter 6). Focal epileptic myoclonus (FEM) is usually restricted to a distal group of muscles; it is the result of an epileptic discharge involving the motor cortex (Chauvel et al., 1992b; Hallett, 1985). The focal jerks can be either spontaneous, or they may be evoked by sensory stimulation, such as tapping, muscle stretching, or electric shocks. In some patients, the jerks can rhythmically recur, producing a sort of tremor (Guerrini et al., 2001). Spontaneous or reflex intermittent focal myoclonus may be associated with focal motor seizures (Chauvel et al., 1978; Sutton and Mayer, 1974). Each jerk represents the discharge of a small group of cortical motoneurons that is somatotopically connected to a group of contiguous muscles. Frequently, the EEG correlate of FEM can be detected only by using jerk-locked (EEG or magnetoencephalogram [MEG]) averaging. FEM can also be classified as positive or negative (Guerrini et al., 1993b).
Focal Epileptic Negative Myoclonus
Epileptic negative myoclonus (ENM) is defined as a brief, jerky, involuntary movement due to muscular silent periods lasting less than 400 ms that is time-locked to paroxysmal EEG activity (usually a spike-wave [SW] discharge) in the contralateral sensorimotor cortex. The atonic phenomenon may not be preceded by a myoclonic jerk (Tassinari et al., 1998; Guerrini et al., 1993b). Focal ENM is usually manifested as jerky movements of the outstretched arm(s) or leg(s). In small children, the involvement of axial segments may produce an ataxic-like picture. The affected children should be tested during the maintenance of a tonic contraction, because the SW discharges are not associated with clinical manifestations in the relaxed muscle.
In some children, severe negative myoclonus may reduce the motor initiative in the affected body part, resulting in a sort of motor neglect. The ENM can also be multifocal (Guerrini et al., 1993b; Kanazawa and Kawai, 1990) or generalized.
Inhibitory Seizures
Focal inhibitory seizures, or partial atonic seizures (PASs), represent a rare seizure type (Guerrini et al., 2002d;
Hanson and Chodos, 1978; Gastaut and Broughton, 1972) characterized by ictal paresis or paralysis of one body segment that is sometimes preceded or accompanied by numbness. Gastaut and Broughton (1972) identified the following two types of PASs: unilateral atonic seizures involving one hemibody and somatic inhibitory seizures with focal distribution. The associated manifestations include deviation of the eyes or head toward the side of the paralyzed limb (Thomas et al., 1998; Globus et al., 1982; Waltregny et al., 1969), clonic jerks in a different body part (So, 1995; Penfield and Jasper, 1954), or dysphasia and/or aphasia (Globus et al., 1982; Fisher, 1978). PAS can be difficult to differentiate from ictal or postictal paresis (Hanson and Chodos, 1978). The duration of PAS ranges from a few seconds to hours. Both children and adults have been reported to present with prolonged seizures (i.e., lasting more than 30 minutes) that can be considered “nonconvulsive epileptic status.”
Hanson and Chodos, 1978; Gastaut and Broughton, 1972) characterized by ictal paresis or paralysis of one body segment that is sometimes preceded or accompanied by numbness. Gastaut and Broughton (1972) identified the following two types of PASs: unilateral atonic seizures involving one hemibody and somatic inhibitory seizures with focal distribution. The associated manifestations include deviation of the eyes or head toward the side of the paralyzed limb (Thomas et al., 1998; Globus et al., 1982; Waltregny et al., 1969), clonic jerks in a different body part (So, 1995; Penfield and Jasper, 1954), or dysphasia and/or aphasia (Globus et al., 1982; Fisher, 1978). PAS can be difficult to differentiate from ictal or postictal paresis (Hanson and Chodos, 1978). The duration of PAS ranges from a few seconds to hours. Both children and adults have been reported to present with prolonged seizures (i.e., lasting more than 30 minutes) that can be considered “nonconvulsive epileptic status.”
Subdural recordings have shown that the seizure activity involves the mesial frontal or the primary sensorimotor cortex (Matsumoto et al., 2000; Noachtar and Lüders, 1999; Hanson and Chodos, 1978). Electrical stimulation studies suggest that “negative motor areas” in the inferior frontal gyrus (primary negative motor area) and anterior to the mesial portion of the superior frontal gyrus (supplementary negative motor area) (Lim et al., 1994) inhibit voluntary movement as long as they are activated (Lüders et al., 1995). Epileptic activity involving these areas can lead to apraxia and motor inhibition that is manifested as PAS (Lüders et al., 1995). However, epileptic discharges in the primary sensorimotor cortex could also produce negative motor phenomena via the direct inhibition of the spinal motoneuron (Matsumoto et al., 2000).
Most paroxysmal attacks of paralysis in childhood are not of epileptic origin, and the diagnosis of “inhibitory seizure” can be accepted only when strong EEG evidence is available.
Drop Attack Seizures in Focal Epilepsy
Drop attack seizures may be the sole or main ictal manifestation in some patients. They may apparently be indistinguishable from generalized tonic or atonic seizures (Dravet et al., 1997; Tassinari et al., 1997) or from epileptic spasms. Two main types of drop attacks occur in focal seizures (Rubboli et al., 1997). One type is characterized by an initial stiffening or tonic posturing and is thought to result either from the involvement of the frontal or supplementary motor areas (Broglin et al., 1992; Waterman and Wada, 1990; Delgado-Escueta et al., 1987; Geier et al., 1977) or from a pathologic startle response in children with startleinduced epileptic seizures. Versive phenomena and vocalization may also be present. Although ictal involvement of the frontal cortex appears to be essential in producing the changes in muscle tone and posture causing this type of drop attack, posterior seizure onset that often is bilateral, followed by anterior propagation, is not uncommon (Biraben and Chauvel, 1997). A second type of epileptic drop attack is characterized by a sudden, possibly atonic, fall without preceding motor phenomena (Pazzaglia et al., 1985; Gambardella et al., 1994), followed by confusion and reduced responsiveness for about 2 to 3 minutes. Such attacks are often associated with temporal lobe phenomena, and they have also been termed temporal lobe syncopes. Depth electrode studies in one patient showed ictal onset in the amygdala and hippocampus, with subsequent rapid spread to the contralateral hippocampus and frontoorbital areas bilaterally (Gambardella et al., 1994). Accurate characterization of the focal origin of the epileptic drop attacks may be important when surgery is contemplated.
SENSORY SEIZURES
Somatosensory Seizures
Somatosensory seizures can originate from any of the following three sensory areas of the parietal lobe: the primary sensory area on the postcentral gyrus (Brodmann areas 1, 2, 3a, and 3b), the second sensory area on the superior border of the sylvian fissure, and the supplementary sensorimotor cortex in the mesial superior frontal cortex (Bleasel and Morris, 1996; Lüders et al., 1985; Martin, 1985). Clinical symptoms resulting from ictal activity in these brain areas are usually referred to the contralateral half of the body, but bilateral involvement is possible. The somatosensory symptoms can spread during the seizure, often in a jacksonian way, starting from the face or an extremity, but they rarely affect the whole half body (Mauguière and Courjon, 1978). Concomitant motor activity occurs in about 50% of sensory seizures with jacksonian march (Sveinbjornsdottir and Duncan, 1993).
Elementary paresthesias (i.e., prickling or tingling sensations, pins and needles, a sensation of something crawling under the skin, or numbness) are the most frequent ictal symptoms (Mauguière and Courjon, 1978; Ajmone Marsan and Goldhammer, 1973). The duration of sensory symptoms is brief, usually lasting less than 1 or 2 minutes (Mauguière and Courjon, 1978).
Most somatosensory seizures are related to the involvement of the postrolandic primary sensory area (Mauguière and Courjon, 1978). Paresthesias and numbness originating in this area usually have a somatotopic distribution and spread that is consistent with a somatotopic representation (Critchley, 1966). The sensation is contralateral, usually involving the hands and the face, and it has a good localizing and lateralizing value (Mauguière and Courjon, 1978). Involvement of the supplementary sensorimotor area is usually characterized by imprecise paresthetic, often bilateral, sensations involving large proximal body regions. Patients often report a general body aura or nonspecific sensations of the trunk (Tuxhorn and Kerdar, 2000). The second sensory area can produce bilateral sensory seizures with the involvement of more than one body region (Penfield and Rasmussen, 1951).
Most somatosensory seizures are related to the involvement of the postrolandic primary sensory area (Mauguière and Courjon, 1978). Paresthesias and numbness originating in this area usually have a somatotopic distribution and spread that is consistent with a somatotopic representation (Critchley, 1966). The sensation is contralateral, usually involving the hands and the face, and it has a good localizing and lateralizing value (Mauguière and Courjon, 1978). Involvement of the supplementary sensorimotor area is usually characterized by imprecise paresthetic, often bilateral, sensations involving large proximal body regions. Patients often report a general body aura or nonspecific sensations of the trunk (Tuxhorn and Kerdar, 2000). The second sensory area can produce bilateral sensory seizures with the involvement of more than one body region (Penfield and Rasmussen, 1951).
The incidence of somatosensory seizures is low in children. In large series, somatosensory symptoms were reported in 8.5% to 25% of patients experiencing auras (Penfield and Kristiansen, 1951). A much lower incidence was reported by Mauguière and Courjon (1978), who diagnosed somatosensory epilepsy in 1,034 (1.4%) of 9,938 patients with epilepsy. Tuxhorn and Kerdar (2000) observed somatosensory auras in 72 (12%) of 600 consecutive patients undergoing presurgical evaluation. The age range in this subgroup varied from 6 to 61 years, and the mean age at epilepsy onset was 8.8 years.
Painful Seizures and Thermal Sensations
Painful seizures and thermal sensations are much rarer than paresthetic attacks. Ictal pain is often described as severe; it is cramplike in the extremities, and throbbing or stabbing in the head and face. The hands, face, and head are most commonly involved. Headache-like symptoms or abdominal pain are also possible (Young and Blume, 1983). Unilateral painful seizures have been linked with parietal epileptic activity (Young and Blume, 1983; Talairach et al., 1960; Whitty, 1953; Penfield and Rasmussen, 1951). Ictal pain is rare in children, and it should not be considered of psychogenic origin (Trevathan and Cascino, 1988). Sensations purely of cold or heat are exceptional. Bilateral sensations and thermal sensations may be related to the involvement of the secondary sensory area in the parietal operculum (Russell and Whitty, 1953; Penfield and Jasper, 1954).
Sexual sensations in the course of seizures are exceptional, and they never occur before puberty. Body image disturbances are rare (Mauguière and Courjon, 1978; Arseni et al., 1966); they are demonstrable only in older children and adolescents, who variably describe kinesthetic illusions of movement or displacement of a motionless limb (Penfield and Gage, 1933); a feeling of floating, twisting or torsion of a body limb (Epstein, 1967); and illusions of swelling or shrinking of a body part. Unilateral asomatognosia with a sensation of absence of a body part has also been reported. Their presence points to the involvement of the inferior parietal lobe posterior to the primary sensory area (Mauguière and Courjon, 1978; Penfield and Jasper, 1954), most often on the nondominant hemisphere (Hecaen and de Ajuriaguerra, 1952).
Ictal Vertiginous Sensations
Ictal vertiginous sensations are not uncommon, and they are often related to disturbances in body image (Smith, 1960; Lennox and Cobb, 1933). Ictal epileptic vertigo can be isolated, or it can precede loss of consciousness. It is usually brief, and it is never accompanied by nystagmus (Karbowsky, 1982). Smith (1960) noticed a strong association with visuospatial illusions and somatosensory sensations.
VISUAL SEIZURES
Visual Hallucinations
Visual hallucinations are the most frequent symptoms; these include bright, colorful, multicolored or occasionally dark rings or spots or simple geometric forms that are continuous or flashing. They are usually, but not necessarily, in the periphery of the visual field contralateral to the ictal discharge and rotate or move slowly to the opposite side (Guerrini et al., 1994, 1995; Williamson et al., 1992b; Bancaud, 1969; Penfield and Jasper, 1954; Russell and Whitty, 1953). Ictal amaurosis, blindness, or severe blurring of vision that is limited to one hemifield or quadrant or involves the entire visual field may follow the visual hallucinations; occasionally, however, it may be the first symptom (Salanova et al., 1992; Williamson et al., 1992b; Bauer et al., 1991; Huott et al., 1974). Hemianopia and quadrantanopia have high localizing value. Testing visual avoidance is important when the clinician witnesses a patient experiencing visual symptoms, both ictal and postictal.
More complex visual hallucinations include scenes often related to past experiences. They may be accompanied by macropsia, micropsia, or perception of scenes of people or animals described as static, moving horizontally, approaching, or moving away
(Sveinbjornsdottir and Duncan, 1993; Williamson et al., 1992b; Blume, 1991). Hallucinations may also include letters or numerals (Sowa and Pituck, 1989; Gastaut and Zifkin, 1984). Older children are usually aware of the hallucinatory nature of the perception. Ictal activity producing complex hallucinations involves the occipital association cortex and the posterolateral temporal cortex.
(Sveinbjornsdottir and Duncan, 1993; Williamson et al., 1992b; Blume, 1991). Hallucinations may also include letters or numerals (Sowa and Pituck, 1989; Gastaut and Zifkin, 1984). Older children are usually aware of the hallucinatory nature of the perception. Ictal activity producing complex hallucinations involves the occipital association cortex and the posterolateral temporal cortex.
Visual Illusions
Visual illusions involving part or the whole of the visual field may be experienced during occipital lobe seizures (Sveinbjornsdottir and Duncan, 1993). They may consist of alterations in the size, shape, or motion of objects or a change in color quality with monochrome vision or lack of color (achromatopsia). More complex illusions may result in altered perception of objects in space. Ictal palinopsia, or the persistence or recurrence of visual images once the real object of perception is no longer present, has been reported (Critchley, 1951; Lefebre and Koelmel, 1989).
Head and Eye Deviation
Visual phenomena are often accompanied or followed by “conscious” tonic or, in rare instances, clonic eye or eye and head deviation that is usually, but not always, toward the side of the initial visual symptoms (contralateral to the side of seizure origin) (Guerrini et al., 2000d; Williamson et al., 1992b; Furman and Crumrine, 1990; Kanazawa et al., 1989; Munari et al., 1984; Bancaud, 1969). Determining whether eye and head turning is part of the seizure or if it is related to the patient’s attempts to follow the images and hallucinatory figures may be impossible. However, ictal eye deviation of occipital origin has an initial tonic phase that is followed by a clonic phase with eye jerks. Oculoclonic movements were defined by Gastaut and Roger (1955) as epileptic nystagmus.
Ictal Manifestation Resulting from Extraoccipital Seizure Propagation
The patients’ ability to recall the visual symptoms points to the initial localization of the ictal discharge near the calcarine fissure, followed by a slow propagation to adjacent areas. When the discharge is occipitotemporal from the onset, the visual phenomena usually cannot be recalled (Munari et al., 1993).
Infrasylvian propagation to the mesiotemporal limbic structures is common (Olivier et al., 1982; Ajmone-Marsan and Ralston, 1957); it is accompanied by automatisms typical of temporal lobe epilepsy (Salanova et al., 1992; Williamson et al., 1992b; Takeda et al., 1969; Bancaud et al., 1961). Some children experience vomiting in the course of prolonged visual seizures (Guerrini et al., 1994, 1995). Propagation to the lateral occipital cortex and temporal neocortex is responsible for complex visual and auditory hallucinations (Munari et al., 1993; Geier et al., 1973; Penfield and Perot, 1963). Suprasylvian propagation to the primary motor cortex is accompanied by focal motor or hemiclonic activity and propagation to the supplementary sensorimotor cortex, by asymmetric tonic posturing (Williamson and Spencer, 1986; Babb et al., 1981; Takeda et al., 1969). Secondary generalization is common when suprasylvian spread occurs.
Ajmone Marsan and Ralston (1957) observed that infrasylvian spread occurs more often when the seizure origin is below the calcarine fissure, whereas suprasylvian spread is most often related to supracalcarine onset. This pattern was not confirmed in another study (Aykut-Bingol et al. 1998). One-third of all patients have more than one seizure type, which indicates multiple possible spread patterns (Aykut-Bingol et al., 1998; Salanova et al., 1992; Williamson et al., 1992b; Bancaud et al., 1965). The manifestations of seizure spread are often the most prominent clinical feature, and these tend to overshadow the visual symptoms (Williamson et al., 1997).
AUDITORY SEIZURES
Auditory seizures or auras originate from the primary and association auditory cortices. Detailed clinical studies on auditory seizures are rare, and no study has specifically addressed their clinical characteristics in children.
Clinical manifestations include elementary and complex hearing symptoms (Foldvary et al., 2000). The elementary symptoms usually include ringing, buzzing, chirping, or humming noises. Complex auditory symptomatology include illusions, with alterations in perception of distance or loudness or temporal characteristics and hallucinations of voices, music, or meaningful sounds that may be referred to a specific source or that may be more vaguely identified (Gloor, 1990; Hurst and Lee Soo, 1986; Wieser, 1983b). The symptoms are referred to one or both ears. When they are unilateral, they most often are referred to the ear contralateral to the discharging hemisphere (Hurst and Lee Soo, 1986; Penfield and Perot, 1963; Penfield and Jasper, 1954).
The incidence of initial or isolated auditory symptoms in patients with focal epilepsy varies, ranging
from 1.7% and 7% (Lennox and Cobb, 1933). More recent series, especially those including patients with temporal lobe epilepsy, have reported auditory auras in 1.7% to 16% of patients (Foldvary et al., 2000).
from 1.7% and 7% (Lennox and Cobb, 1933). More recent series, especially those including patients with temporal lobe epilepsy, have reported auditory auras in 1.7% to 16% of patients (Foldvary et al., 2000).
GUSTATORY AND OLFACTORY SEIZURES
Recent studies indicate that about 7.1% of adult patients with partial epilepsy (Manford et al., 1996a) and about 10% of those having sensory auras (Ebner and Kerdar, 2000) experience gustatory and olfactory sensations. In a large series of 222 adults diagnosed with temporal lobe epilepsy, 6.3% had olfactory and gustatory auras (Ebner and Kerdar, 2000). No studies on their prevalence and ictal characteristics in children are available.
Gustatory Seizures
Gustatory seizures are rarely an isolated symptom. Most patients experience hallucinations of taste that are associated with a sensation of smell. Abnormal taste sensations are bitter, acidic, or sweet and usually are disgusting (Sveinbjornsdottir and Duncan, 1993).
Gustatory hallucinations are considered an expression of either parietal-opercular or anterior temporal seizure activity, without lateralizing value.
Olfactory Auras
Olfactory auras are most often a symptom of a mesial temporal origin (Ebner and Kerdar, 2000). However, posterior orbitofrontal origin has also been demonstrated with depth electrodes (Roper and Gilmore, 1995; Bancaud and Talairach, 1992). The quality of the sensation is generally described as disagreeable (Ebner and Kerdar, 2000).
ICTAL AUTONOMIC PHENOMENA
Autonomic symptoms may be predominant in some children (Afifi et al., 1990; Coulter, 1984; Marshall et al., 1983); they may include respiratory (Southall et al., 1987; Davis et al., 1986), cardiovascular (Davis et al., 1986), pupillary (Wieser, 1987), gastrointestinal (Jacome and Fitzgerald, 1982), sudomotor, pilomotor, and salivatory manifestations. They may occur as isolated symptoms in a fully aware child, or, more often, they may be part of a more complex seizure symptomatology in an unconscious child. They may sometimes be prominent during the motor seizures that are typical of childhood, especially unilateral clonic seizures (Dravet, 1992a). Some autonomic auras represent the subjective awareness of a change in the activity of the autonomic nervous system (So, 1993). Others consist of objective changes in autonomic function (O’Donovan et al., 2000). Often, autonomic manifestations are reported by the parents of children with epilepsy, who describe seizures with alteration of consciousness and “dilated pupils,” “pallor, or flushing around the mouth.” Some degree of hemispheric lateralization of autonomic functions does exist, with the right hemisphere having a predominant effect (O’Donovan et al., 2000), but the localizing and lateralizing value, if any, is limited. In addition, autonomic changes during a seizure may result from the child’s anxiety reaction to experiential symptoms (Ledoux, 1992; Gloor et al., 1982b).
Cardiovascular symptoms include tachycardia and bradycardia, arrhythmias, hypertension, flushing, or pallor. Bradycardia and arrhythmogenic seizures are rare (Zelnik et al., 1990; Davis et al., 1986; Gilchrist, 1985); they may sometimes be of a dramatic character that may be a cause of the sudden infant death syndrome (Smaje et al., 1987; Kiok et al., 1986; Southall et al., 1985). Both limbic and extratemporal areas, including the cingulate, orbitofrontal cortex, and amygdala, may be involved (O’Donovan et al., 2000; Anand and Dua, 1956). According to Stodieck and Wieser (1986), the slowing of the heart rate correlates with discharges from the amygdala.
Respiratory symptoms may present as short respiratory arrest, deep inspiration at seizure onset, or apnea or hyperpnea (Monod et al., 1988; Coulter, 1984) accompanying partial seizures (Wieser et al., 2000). Stimulation studies indicate that the mesiobasal limbic structures are involved in causing ictal apnea (Wieser, 1983b; Nelson and Ray, 1968).
Pupillary manifestations with mydriasis or myosis are a common symptom accompanying the arrest reaction (Wieser et al., 2000). The mydriasis can be asymmetric (Wieser, 1987).
Gastrointestinal symptoms include the classic epigastric aura or rising epigastric sensation of mesial temporal lobe epilepsy, eructation, borborygmus, nausea, and vomiting. The so-called abdominal epilepsy is one possible manifestation of limbic involvement. It features abdominal discomfort or pain that may be periumbilical, epigastric, or poorly localized, and it is often accompanied by disturbances in gastrointestinal motility and/or vomiting (Mitchell et al., 1983). Abdominal manifestations rarely occur in complete isolation without a subsequent loss of consciousness or automatisms. The authors have seen children suffering from abdominal seizures, with or without loss of awareness, due to temporal ganglioglioma,
who were treated for several years in gastroenterology units for gastroesophageal reflux. Isolated vomiting or ictus emetica (Thomas and Zifkin, 1999; Devinsky et al., 1995; Guerrini et al., 1994; Panayiotopoulos, 1988; Fiol et al., 1988; Kramer et al., 1988; Jacome and Fitzgerald, 1982) is rare. The link between abdominal aura and mesial temporal lobe epilepsy represents the most reliable association of an autonomic aura with the area of seizure onset.
who were treated for several years in gastroenterology units for gastroesophageal reflux. Isolated vomiting or ictus emetica (Thomas and Zifkin, 1999; Devinsky et al., 1995; Guerrini et al., 1994; Panayiotopoulos, 1988; Fiol et al., 1988; Kramer et al., 1988; Jacome and Fitzgerald, 1982) is rare. The link between abdominal aura and mesial temporal lobe epilepsy represents the most reliable association of an autonomic aura with the area of seizure onset.
Ictal pilomotor and sudomotor manifestations are very rare. Sudomotor manifestations, especially if they are accompanied by tachycardia and fear, are a common symptom of panic attacks (Guerrini et al., 1998b).
Genitourinary or sexual sensations are exceptional in children. Feindel and Penfield (1954) noted that 8% of their patients with temporal lobe epilepsy reported a “desire to void” as their initial ictal symptom.
COGNITIVE SEIZURES
Paroxysmal disturbances of cognitive functions are a common feature of a number of focal epilepsies, and they are further considered (see “Focal Seizures Characterized by Prominent Disturbances of Consciousness with or without Automatisms and Cognitive and Affective Manifestations”). In such cases, the cognitive disturbances may constitute the “aura” of a more complex seizure event. In less common cases, cognitive dysfunction constitutes the whole seizure, and the term cognitive seizures is more specifically applied to such cases. This section considers all cases in which cognitive disturbances are a prominent component of the seizures, irrespective of their association with other ictal events, such as disturbances of consciousness, automatisms, or other motor or autonomic phenomena. Isolated impairment of consciousness is not included. Paroxysmal affective changes often accompany cognitive phenomena, and they contribute to both their clinical presentation and the subjective experience of the patients. They are indeed often impossible to analyze separately from the cognitive manifestations, and the previously proposed term affective-psychic seizures is probably justified (Aicardi, 1994a).
Dreamy State
The dreamy state first described by Hughlings Jackson is the most classic form of cognitive seizure. It involves a disruption in the perception of reality that affects both time and memory processes. The ictal state interferes with ongoing cognition, producing what Jackson called “double consciousness.” The dreamy state is associated with dysmnesic symptoms, which consist of illusions of recall that the patient cannot separate from his or her current experience. Best known is the “déjà vu” feeling in which the patient believes that he or she is recognizing and living again a previous experience; the converse sensation of unfamiliarity and strangeness may, however, also occur (jamais vu feeling). Hallucinations that include vivid reminiscences of past experiences, the experiential hallucinations of Penfield and Jasper (1954) and Penfield and Perot (1963) are often part of the dreamy state. During these episodes of abnormal mental state, a considerable degree of awareness may be preserved, and the patient may be able to perform complex acts requiring the use of normal faculties (Jackson quoted by Aicardi, 2001a), although these acts have not been memorized. The dreamy state seems to require temporal lobe involvement (especially of the right) in the discharge.
Forced Thinking
Forced thinking is a rare ictal manifestation in which a thought unrelated to the current situation imposes itself on the patient’s mind (Reinikainen, 1987; Broglin et al., 1992); it seems to be observed with frontal lobe discharges. Multiple other cognitive dysfunctions are also present. They are described in the section on disturbances of consciousness, with which they are often associated.
Other Manifestations
One interesting manifestation of cognitive seizures is the transient and isolated disturbance of selective mental processes, resulting in errors or slowness in the execution of specific mental tasks thought to affect localized cortical areas involved by the epileptic discharge (e.g., visuospatial tasks with discharges in the right parietooccipital area and verbal tasks with those affecting the left frontotemporal hemisphere). Such paroxysmal dysfunctions may be very brief, and they may go undetected. Recognizing these may require EEG recording during the performance of continuous tasks (Kasteleijn-Nolst Trenité et al., 1988, 1990; Shewmon and Erwin, 1988; Tsuchiya et al., 1978). These so-called transient cognitive impairments (TCI) are often ignored by the patient, but they can, in some circumstances, significantly disturb their life. This can also be the case with discharges in areas involved in amnesic recall, as in the patient reported by Aarts et al. (1984), who could not retrieve
the information required by his profession of librarian, demonstrates. Interestingly, this occurred only when he was under stress, and the EEG at rest was normal; it did show SW activity only when he was challenged. The possible role of SW activity unassociated with clinical seizures has been proposed as an explanation for deterioration in some epilepsy syndromes, such as Landau-Kleffner and continuous spike-waves of slow sleep (CSWS) (Aicardi, 1999a; Deonna, 1996) (see Chapter 11).
the information required by his profession of librarian, demonstrates. Interestingly, this occurred only when he was under stress, and the EEG at rest was normal; it did show SW activity only when he was challenged. The possible role of SW activity unassociated with clinical seizures has been proposed as an explanation for deterioration in some epilepsy syndromes, such as Landau-Kleffner and continuous spike-waves of slow sleep (CSWS) (Aicardi, 1999a; Deonna, 1996) (see Chapter 11).
Aphasic seizures are rare in children. The most common aphasia associated with epilepsy is Landau-Kleffner syndrome (Chapter 11). Ictal aphasia has been well described in adults (Wells et al., 1992). In children, however, isolated ictal aphasia has not been reported. Speech arrest and especially aphemia are common with seizure discharges involving the operculum in rolandic epilepsy (RE), but this is rarely isolated. Ictal aphasia should be distinguished from the more common confusion and postictal aphasia that may follow temporal lobe seizures.
FOCAL SEIZURES CHARACTERIZED BY PROMINENT DISTURBANCES OF CONSCIOUSNESS, WITH OR WITHOUT AUTOMATISMS OR COGNITIVE AND AFFECTIVE MANIFESTATIONS
This section deals with focal epileptic seizures that feature alterations or abolition of consciousness in the absence of generalization of the attack. This section first considers the general characteristics and pathophysiology of these seizures and then the effect of age, their different clinical expressions, and their diagnosis.
Alteration of Consciousness
Historical Note and Definitions
Disturbances of consciousness during partial seizures have long attracted the interest of neurologists, and their association with cognitive or affective manifestations has been known for a very long time.
In 1937, Gibbs et al. (1937) used the term psychomotor to encompass all the psychic and motor manifestations encountered in partial seizures in which alteration of consciousness was a prominent feature, but the same term was subsequently applied to automatisms as a specific ictal manifestation (Penry, 1975). The discovery that many of the seizures presenting with such phenomena originated in the temporal lobe, especially its limbic part (Lennox and Lennox, 1960; Penfield and Jasper, 1954), and that they were often associated with anterior temporal spike foci (Gibbs et al., 1937) led to the introduction of the term temporal lobe seizures to designate the same type of attacks, even though a possible origin from other sites was quickly recognized (Penfield and Jasper, 1954; Ajmone-Marsan and Abraham, 1960). The terms psychomotor seizures, temporal lobe seizures, and limbic seizures have often been used synonymously regardless of the fact that they refer to different conceptual categories (topographic, descriptive, EEG), thereby producing increasing confusion. The term complex partial seizures was introduced by the International Classification of Epileptic Seizures in 1970 (see Chapter 2), which hoped to end the confusion by selecting the clinical and EEG manifestations as the main basis for classifying the seizures. The 1970 classification, however, used the term complex partial seizures synonymously with temporal lobe seizures. Subsequent studies addressing the anatomic substrate(s) of clinical seizure semiology, which were mainly derived from studies for epilepsy surgery, have clarified that both temporal and extratemporal seizures may be associated with alteration of consciousness. The 1981 revision of the International Classification of Epileptic Seizures, while retaining the distinction between simple and complex partial seizures based on the presence of alteration of consciousness, did not assign alteration of consciousness to any specific anatomic substrate. Additional evidence gathered during the last 20 years has led to the belief that the designation of partial seizures as “simple” or “complex” has lost meaningful precision for classifying seizures in terms of topography; pathophysiology; and, at times, clinical semiology (Engel, 2001). Consequently, the distinction “simple” versus “complex” is no longer recommended.
Because of the close, although not necessary, association between automatisms and alteration of consciousness, this section also includes some discussion of the automatic motor activities and subjective symptoms more commonly associated with and essential to the understanding of the phenomenology of seizures with alteration of consciousness. More details about clinical semiology of automatisms and affective psychic phenomena are provided in specific subsequent sections of this chapter.
Isolated impairment of consciousness was recognized as a distinct type of partial seizure by the 1989 international classification. Such attacks consist of a brief episode of confusion for which the patient is subsequently partially or entirely amnesic (Daly, 1982). In most of these seizures, a few inconspicuous automatisms likely occur. Such seizures differ from absences by their longer duration (30 to 90 seconds);
their more gradual termination; and the presence of some postictal tiredness and sleepiness, which is a major distinctive characteristic in children (Penry et al., 1975). From a purely semiologic perspective, the term dialeptic seizures (Lüders et al., 2000; Noachtar et al., 2000) has been suggested to define the ictal alteration of consciousness that is accompanied by staring and a loss or minimal persistence of motor activity, regardless of its pathophysiology (i.e., focal versus generalized).
their more gradual termination; and the presence of some postictal tiredness and sleepiness, which is a major distinctive characteristic in children (Penry et al., 1975). From a purely semiologic perspective, the term dialeptic seizures (Lüders et al., 2000; Noachtar et al., 2000) has been suggested to define the ictal alteration of consciousness that is accompanied by staring and a loss or minimal persistence of motor activity, regardless of its pathophysiology (i.e., focal versus generalized).
In the same semiologic classification (Lüders et al., 2000), the term hypomotor seizures was suggested for seizures with a significant reduction in or arrest of behavioral motor activity occurring in small children or severely mentally retarded individuals in whom an appreciation of the level of consciousness during an attack is impossible (Acharya et al., 2000; Wyllie, 1995). The term akinetic was suggested for seizures in which a patient is unable to follow a simple motor command but retains full recall of the event (Noachtar and Lüders, 2000). An advantage of this proposal is that the level of “consciousness,” which is so difficult to assess in young patients, need not be defined.
Other seizures that often alter “consciousness” are characterized by the predominance of motor activity, and different terms can be used according to the specific type of activity (e.g., “automotor” in the presence of automatisms, “clonic” when rhythmic jerks occur). In the present chapter, the authors do not use these terms because they are not generally accepted.
Automatisms (see “Automatisms”) are behavioral sequences reproducing normal body movements that unfold without voluntary control and that, in a majority, occur with a loss or impairment of awareness. They occur with different types of seizures, whether generalized or partial, or as postictal manifestations (Talairach et al., 1974). Simple automatisms, especially of those of an oral alimentary type, can occur without impairment of consciousness (Munari et al., 1980a). Seizures in which distal automatisms are prominent are also described as “automotor” seizures (Kotagal, 2000), and these are considered highly characteristic of mesial temporal lobe epilepsy. Automatisms also occur in seizures arising from the frontal, parietal, and occipital lobes (Salanova et al., 1995b; Munari et al., 1980a; Geier et al., 1976).
Affective-psychic ictal phenomena are considered simple partial seizures in the 1981 classification because consciousness is often not impaired, even though the ictal events are highly complex. The state of consciousness during such seizures is often modified in a subtle way that is not necessarily part of the operational definition given. Affective-psychic phenomena often evolve into loss of consciousness.
In summary, focal epilepsies with alterations of consciousness have long been regarded as a relatively homogeneous disorder. However, they clearly constitute a heterogeneous group with different sites of origin of the discharges (Wieser, 1983b; Talairach and Bancaud, 1974). Two major categories are represented by seizures originating in the limbic system, especially its temporobasal part, which includes the hippocampus, amygdala, and adjacent temporofrontal cortex, and those originating from the neocortex, whether in the temporal lobe or outside it, especially the frontal lobe. These two groups have different clinical manifestations (Engel, 1987, 1992; Quesney, 1987; Wieser, 1987) and different courses, and they pose different therapeutic problems.
Pathophysiology of Alteration of Consciousness in Focal Seizures
Alteration of consciousness in focal seizures can be produced by at least the following four mechanisms: (a) widespread diffusion of the ictal discharge to the cortex, which consequently is diffusely inactivated (Bancaud and Talairach, 1992); (b) spreading of the discharge to the upper brainstem (Noachtar et al., 2000); (c) inactivation of the hippocampal formation bilaterally, with a consequent inability to store memories (Lüders et al., 2000); and (d) involvement of one or more of the language areas (Broca, Wernicke, or basal temporal area), leading to global aphasia (Lüders et al., 1987a, 2000; Lesser et al., 1986). These mechanisms may operate independently, or they may be combined in various proportions.
A fundamental concept is that alteration of consciousness is indicative of extensive seizure diffusion to the limbic or neocortical temporal and extratemporal areas and that it therefore reflects the amount of cortex involved but does not indicate per se any specific area of seizure origin or topographic distribution (Munari et al., 1980a). Therefore, alteration of consciousness is not particularly important for seizure localization, but it is a factor of major importance when assessing the level of seizure-related disability.
Neurophysiologic analyses of alteration of consciousness in focal seizures have been conducted mainly in patients with frontal and mesial temporal epilepsies. Electrical stimulation of the mesial frontal lobe can elicit “frontal absences” and generalized SW discharges on scalp EEG (Bancaud et al., 1974). Spontaneous ictal activity in the mesial frontal lobe
may be responsible for a blank staring ictal symptomatology (Wieser, 1987). Electrical stimulation studies of the frontal lobes have also identified areas that can produce motor arrest (Lüders, 1992). Close connections linking the prefrontal cortex with nonspecific and intralaminar thalamic nuclei of the midline region might facilitate rapid ictal spread from the anterior frontal lobes to the reticular formation, causing alteration of consciousness and generalization (Noachtar et al., 2000).
may be responsible for a blank staring ictal symptomatology (Wieser, 1987). Electrical stimulation studies of the frontal lobes have also identified areas that can produce motor arrest (Lüders, 1992). Close connections linking the prefrontal cortex with nonspecific and intralaminar thalamic nuclei of the midline region might facilitate rapid ictal spread from the anterior frontal lobes to the reticular formation, causing alteration of consciousness and generalization (Noachtar et al., 2000).
Most focal seizures with loss of consciousness appear to originate in the temporal lobe(s), especially the limbic formation and various areas of the neocortex within the temporal lobe(s); the frontal lobe(s); or other areas. The limbic structures consist of the following three major interconnected neuronal constellations, each of which is centered around a particular group of nuclei: (a) the frontotemporal limbic cortex and the amygdala, through which impulses come from and go to the brainstem; (b) the hippocampal and parahippocampal cortex and the septal nuclei; and (c) the cingulate cortex and the anterior thalamus (Koella, 1987; Pandya and Yeterian, 1987). From animal experiments, these structures are thought to subserve autonomic and behavioral functions related to individual and species preservation, although the evidence in humans is far from complete (Koella, 1987). They are involved in the elaboration of behavioral responses to external stimuli, as well as corresponding affects and internal sensation, and integrate at the highest level most autonomic functions.
The temporal neocortical part of this system plays an essential role in integrating the sensory inputs from primary receiving areas in order to elaborate perceptions, then to match them with previous experience, and finally to evaluate whether they are motivationally meaningful for the individual (Gloor et al., 1982b). The mesial temporal structures also play an essential role in the mechanisms of memory. Disruption of this system by an epileptic discharge results in a distortion of perceptions, inappropriate associated affects, and inability to match ongoing perceptions to previous experience, thus disturbing the evaluation of a perception as novel or strange. Automatic motor activity, which may accompany alteration of consciousness, may result from the involvement of certain motor nuclei (e.g., the amygdaloid complex in oral alimentary automatisms), or it may be a release phenomenon caused by bilateral diffusion of the discharge (Wieser and Kausel, 1987) or a postictal phenomenon.
Seizures with alteration of consciousness are a dynamic process. Depth electrode studies during seizures have shown paroxysmal activity in several limbic system areas (cingulate gyrus, amygdala, hippocampus) and extralimbic sites (selected thalamic nuclei and various neocortical areas in or outside of the temporal lobes) (Wieser and Elger, 1987; Engel et al., 1981; Spencer, 1981; Bancaud et al., 1973). Determination of the actual site of origin of the discharge necessitates the convergence and compatibility of several of the following types of data: clinical manifestations; surface electrode and intracranial electrode findings; electrical stimulation studies; neuroradiologic evidence; positron emission tomographic (PET) scans; and SPECT scan studies, if they are available (Krakow et al., 1999; Engel, 1982, 1992; Theodore et al., 1983a, 1984a, 1988, 1990; Bancaud et al., 1973). The discharge migrates from its site of origin to neighboring or distant homolateral and contralateral structures. Preferential pathways of propagation can then be recognized (Wieser et al., 1993; Wieser and Elger, 1987; Wieser and Müller, 1987; Talairach and Bancaud, 1974). For example, Wieser and Kausel (1987) found that most limbic seizures have a focal or regional unilateral mesiobasal onset that usually involves both the hippocampus and the amygdala with spread to the contralateral hippocampal formation. Conversely, some seizures that originate in other brain areas such as the posterior, temporal, parietal, occipital, or frontal lobes often spread to the ipsilateral mesiobasal-limbic structures, which can then act as secondary “pacemaker zones” (Wieser and Müller, 1987), further distributing and maintaining the ictal event. The sequence of the symptoms and of EEG phenomena should therefore be carefully analyzed when surgery is contemplated.
Clinical Manifestations of Focal Seizures with Ictal Alteration of Consciousness as the Main Characteristic
Seizures with alteration of consciousness can manifest with a variety of associated symptoms that may differ considerably with the site of origin and propagation of the ictal activity, the age of the patient, and the nature and extent of the causative lesions. The clinical features of focal seizures with alteration of consciousness have been well defined in adults (Kotagal, 2001; Engel, 1992; Van der Wens and Binnie, 1987; Wieser and Kausel, 1987; Munari et al., 1982b; Bancaud, 1973), but information about their characteristics in children is less abundant (Mohamed et al., 2001; Acharya et al., 2000; Hamer et al., 1999; Wyllie et al., 1989; Wyllie and Lüders, 1989; Duchowny, 1987; Dinner et al., 1984; Holmes, 1984; Holowach et al., 1961).
Peculiarities of Seizures with Alterations of Consciousness in Infants and Young Children
The level of consciousness during seizures in infants and small children or in individuals with mental retardation is difficult to assess. Duchowny (1987) acknowledged that alteration of consciousness is difficult to demonstrate and that it often is assumed only on the basis of unsuccessful attempts to draw attention during an episode (Acharya et al., 1996). Therefore, using the objective term hypomotor to designate seizures, both focal and generalized, with a prominent reduction of behavioral motor activity might seem preferable. These authors studied seizure semiology in 23 children from 2 to 24 months of age who had focal epilepsy. Retrospective video-EEG assessment of the level of AC, as tested by the ictal assessment of reactivity to external stimuli, proved unreliable in most patients. A subgroup of children presented with a homogeneous ictal pattern consisting of arrest or a marked reduction of behavioral motor activity lasting a few minutes, followed by the resumption of preictal activity or an increase of activity. None of the children presented with fine motor or complex automatisms, but some did have oral alimentary activity. However, the “hypomotor” ictal behavior may be the consequence of the limited clinical repertoire that is typical of infants, more than it is an expression of bland “complex partial seizures” (Hamer et al., 1999; Nordli et al., 1997). Hamer et al. (1999) reviewed 296 videotaped seizures from 76 children younger than 3 years of age. A total of 81% of the attacks could be classified within four main categories, with 20% being hypomotor seizures. Hypomotor seizures were associated with focal (14 [70%] of 20) or generalized (6 [30%] of 20) ictal EEG activity. Nordli et al. (1997) proposed the term behavioral seizures to designate the abrupt change in behavior that is seen in infants without additional features and that sometimes include the sudden cessation of movement. Recently, Folgarasi et al. (2002) showed that brain maturation significantly affects the seizure semiology. Motor phenomena of multiple types, including spasms and tonic or hypotonic seizures, are more common in infants than are the typical features of temporal lobe origin.
A common sequence in children is the initiation with an aura of abdominal discomfort that often wells up from the belly and tightens the throat or with vague feelings referred to the alimentary tract (e.g., “fear in my stomach”); it continues with a vacant stare and loss of contact, followed by a period of confusion. In small children, behavioral arrest with unresponsiveness and a change in facial expression may represent the whole seizure.
Associated autonomic features are often present. Pallor of the face with cyanosis around the lips and dark circles around the eyes are often observed. Facial flushing is less common. Blume (1989) underlined the predominance of gastrointestinal symptoms, abdominal sensations, and/or fear, which he found in 11 of 22 patients. Initial staring, which has no localizing value, is commonly observed during alteration of consciousness (Wieser and Kausel, 1987). An abrupt loss of consciousness without automatisms is a well-recognized manifestation of some focal epilepsies, mainly in those with a mesial frontal and frontoorbital seizure origin (Chauvel et al., 1995; Chauvel and Bancaud, 1994; Bancaud and Talairach, 1992), and this may be difficult to distinguish from absence seizures of generalized epilepsy. However, seizure characteristics may point to a specific topographic origin (e.g., arrest of speech and movement, simple automatisms, conjugate eye and head deviation, and quick recovery). An abrupt lapse of consciousness and an arrest of movement without automatisms are also seen in patients with temporal lobe seizure onset (Noachtar et al., 2000; Delgado-Escueta and Walsh, 1983). A cluster analysis of ictal symptoms in patients with frontal and temporal lobe seizure origin indicated that ictal alteration of consciousness with minor or no motor activity occurred in both groups and that it could not be differentiated clinically (Manford et al., 1996a). In a study of 34 patients with focal epilepsy in whom arrest of activity and alteration of consciousness (“dialeptic seizures”), documented by video-EEG recordings, were the predominant ictal features, epilepsy was classified as having a temporal lobe origin in 11 patients, a frontal origin in 6, and a parietooccipital origin in 2, but the origin could only be lateralized and not defined further topographically in a large subgroup of 15 patients (Noachtar et al., 2000).
Subjective Symptoms
Auras, which are observed in most adult patients (Kanemoto and Janz, 1989; Taylor and Lochery, 1987; Gupta et al., 1983), are identified in less than one-third of children (Holmes, 1984). The simple partial seizure termed aura is probably present in more patients, but infants and young children cannot describe their feelings; they are, however, suggested by the fact that, before losing consciousness, the child may look panicked or otherwise “abnormal” for a few seconds.
Simple partial onset, the pathophysiologic substrate for subjective symptomatology, can be manifested with a wide range of symptoms.
Psychosensory Symptoms
Psychosensory symptoms include hallucinations (i.e., a perception in the absence of the appropriate stimulus) and illusions (i.e., disturbed perceptions of ongoing stimuli). Depending on the cortical areas involved, hallucinations and illusions can be visual and formed, with figures and scenes; unformed; auditory; vertiginous; olfactory; gustatory; somatosensory; or multimodal. The elaborateness of visual and auditory hallucinations is variable (Daly, 1982; Gastaut and Broughton, 1972). Multimodal hallucinations (Daly, 1982) consist of complex scenes with combined visual and auditory sensations, that often occur in association with affective changes, especially fear.
Cognitive Symptoms or Auras
Cognitive symptoms or auras, or “experiential phenomena,” designate abnormal experiences resulting from dysmnesic (e.g., déjà vu) and cognitive (e.g., dreamy state, distortion of time sense) seizures (Commission on Classification and Terminology of the International League Against Epilepsy, 1981). During electrical stimulation of the “memory cortex, things [that are] seen or heard may seem strangely familiar, or they may seem strange, absurd, terrifying. They may seem suddenly more distant or nearer” (Penfield and Jasper, 1954). The resulting experience, which is usually retrieved from the patient’s personal past, may be even more vivid than when it was experienced in normal life, with a combination of perception, memory, and affect (Gloor, 1990).
Affective Symptoms
Affective symptoms include mainly unpleasant feelings, the most common of which is fear, that are often referred to the epigastric region. The fear is often intense, and it occurs either in isolation (Biraben et al., 2001) or in combination with autonomic or psychosensory symptoms, particularly with hallucinations that may motivate the affect. A frightened expression associated with an epigastric sensation, pallor, or perioral cyanosis is often observed before the child becomes unresponsive. On some occasions, the child may scream loudly with a terrified expression and then run to hug the parents in a state of confusion. Depth-electrode recordings in patients with ictal fear, anguish, or a combination of the two have proved that the epileptic discharge spreads to involve the amygdala and hippocampus, the frontoorbital region, and the anterior cingulate gyrus (Bancaud and Talairach, 1992). Panic attacks of epileptic origin, which are not easily distinguishable from the more common ictal fear, have been associated with right parietal ictal activity (Alemayehu et al., 1995). Depressive feelings or pleasurable sensations are unusual (Holden et al., 1982), and anger is rare, especially in children. Crying or laughing inappropriately may occur (Offen et al., 1976; Gascon and Lombroso, 1971; Loiseau et al., 1971). Both manifestations in children may be more common with diencephalic lesions (see “Symptoms of Lesional Epilepsy of Subcortical Origin”).
Autonomic Symptoms
Autonomic symptoms have been dealt with in a separate section in this chapter. Here, only the abdominal aura, an ictal manifestation often preceding alteration of consciousness, is considered. Abdominal or epigastric auras are the most commonly identifiable subjective symptoms (Kramer and Bracht, 2000). About 30% of patients with seizure onset in the temporal lobe who were able to report an aura described an abdominal sensation (Kotagal, 1991). Abdominal aura is reliably associated with mesiotemporal epilepsy. However, in few patients, an abdominal sensation may be the expression of mesial prefrontal onset.
Automatisms
Automatisms are involuntary, more or less coordinated motor activities that often, but not necessarily, are associated with the impairment of consciousness. Ictal activity confined to the amygdala and anterior hippocampus can cause oral alimentary automatisms without impairment of consciousness (Munari et al., 1981, 1982b). Automatisms may apparently arise without preceding symptoms, or they may represent the extension of an episode with prominent sensory or affective-psychic manifestations. They may also follow simple partial motor seizures, especially with attacks of frontal origin. Most automatisms are relatively simple, and they affect mainly the oral alimentary domain (Theodore, 1983b; Daly, 1982; Ajmone-Marsan and Ralston, 1957). Chewing, smacking, swallowing, lip pursing, and hissing are particularly common in children (Holowach et al., 1961; Glaser and Dixon, 1956). More complex automatisms include looking around, searching, grimacing, fumbling with clothes or sheets, and scratching movements. Verbal automatisms (e.g., humming noises, repetition of phrases or more often of senseless words) may also occur. The most complex automatisms, such as coordinated gestures or ambulation toward some goal, are usually influenced more or less by environmental events. Some automatisms feature violent motor activity, shouting, and emission of profanities, and these are more common with a seizure
of orbitofrontal origin. A large majority of automatisms are simple, primitive, and undirected (Theodore et al., 1984b; Theodore, 1983b). Automatisms during which some interaction is seen between the patient’s activity and the outside world have been termed reactive, whereas those without interaction have been called released or unreactive (Penry, 1975). Gastaut and Broughton (1972) have classified automatisms into the following types: alimentary, mimetic, gestural, ambulatory, and verbal.
of orbitofrontal origin. A large majority of automatisms are simple, primitive, and undirected (Theodore et al., 1984b; Theodore, 1983b). Automatisms during which some interaction is seen between the patient’s activity and the outside world have been termed reactive, whereas those without interaction have been called released or unreactive (Penry, 1975). Gastaut and Broughton (1972) have classified automatisms into the following types: alimentary, mimetic, gestural, ambulatory, and verbal.
Ictal automatisms must be distinguished from postictal automatic behavior, which can arise in a state of confusion or which can be the expression of residual localized ictal activity (Devinsky et al., 1994). The postictal state may often not be readily apparent, and postictal automatic behaviors are usually of a different nature; they consist of getting up, walking, or running (Kotagal, 2000).
Ictal automatisms result from the activation of specific brain structures or represent release phenomena (Jasper, 1964). Stereotypic oral alimentary and hand automatisms only occur ictally, supporting the hypothesis that at least some automatic activities are the expression of direct epileptic activation of codified behavioral sequences. Stimulation studies have shown that masticatory (oral alimentary) activity, which may be accompanied by nausea or sickness and confusion, results from the direct stimulation of the amygdala and periamygdaloid region but not of the hippocampus (Feindel and Penfield, 1954).
Approximately 40% to 80% of patients with temporal lobe epilepsy have stereotypic automatisms of the mouth and hands, as well as other complex motor manifestations (Kotagal, 2000). In infants and children, oral alimentary activities are predominant, and the automatisms have less purposefulness (Brockhaus and Elger, 1995; Bye and Foo, 1994; Jayakar and Duchowny, 1990; Yamamoto et al., 1987b). More complex manual and gestural automatisms, such as fumbling or picking at clothes, are uncommon, and these tend to occur after 5 years of age (Wyllie et al., 1993). Automatisms were seen in 78% of children 3 to 13 years of age (Yamamoto et al., 1987b) and 87% of those who were 5 to 19 years of age (Holmes, 1984). In newborns and infants up to 2 years of age, the frequency varies from 57% (Holmes, 1984) to 80% (Yamamoto et al., 1987b). In the same age-group, Yamamoto et al., (1987b) observed that, although the location of initial ictal EEG changes was variable, the clinical manifestations were stereotypic, with the most common sequence being represented by motionlessness or simple automatisms, followed by convulsive movements and oral automatisms. Duchowny (1987) observed that the most common automatism in 14 infants younger than 2 years was behavioral arrest with forced head turning and asymmetric tonic posturing that were inconsistently accompanied by sucking, mouthing, and blinking. Holmes (1984) studied 69 complex partial seizures in 24 children. He classified the seizures into the following three basic types: those that began with a motionless stare followed by automatisms (type I); those that began with automatisms (type II); and those (in only one patient) that started with a sudden loss of body tone, followed by confusion, amnesia, automatisms, and gradual recomposure (type III). As in adults (Delgado-Escueta and Walsh, 1985), the type I seizures were usually associated with lateralized ictal discharges, whereas type II and type III seizures were generally associated with bilateral ictal EEG abnormalities. Two additional studies of “complex partial seizures” in children with temporal lobe epilepsy, which was defined by the good seizure outcome after temporal lobectomy (Wyllie et al., 1993) or by EEG and MRI findings (Brockhaus and Elger, 1995), concluded that older children had seizure symptomatology analogous to that of adults but that smaller children had no (retrievable) auras and complex automatisms.
Adolescents and older children can exhibit the full range of automatic activities expressed in adult patients and reviewed in many papers (Lüders et al., 2000; Bancaud, 1987; Munari et al., 1982b; Delgado-Escueta et al., 1977; Ajmone-Marsan and Abraham, 1960). A retrospective cluster analysis of 91 seizures in 31 patients who had EEG monitoring before surgery and were subsequently rendered seizure free after temporal lobectomy showed that oral alimentary and repetitive hand automatisms occurred in 63% of the seizures and that these were correlated with each other and with loss of consciousness and looking around. Auras occurred in 29% of the seizures, an initial behavioral arrest in 36%, and staring in 10% (Kotagal et al., 1995b). Motor phenomena that involved bilaterally the trunk (e.g., flexion, lateral incurvation, rotation) may also occur. Dystonic posturing of one upper limb or of both upper and lower limbs on one side during alteration of consciousness points to a temporal lobe origin in the hemisphere opposite the side involved (Kotagal, 1991; Kotagal et al., 1989). The close temporal relationship between oral alimentary and manual automatisms and dystonic posturing could be due to the involvement of the basal ganglia, as demonstrated on invasive EEG recordings and ictal SPECT studies (Newton et al., 1992; Kotagal et al., 1989). Automatic activity in seizures of temporal lobe origin tends to be predominant or to be limited to the limbs ipsilateral to the involved hemisphere.
The combination of ipsilateral automatisms and contralateral dystonic posturing is highly characteristic of temporal lobe seizures (Kotagal, 1999; Wieser et al., 1993), and it has a good lateralizing value. Unilateral automatisms do not have a lateralizing value on their own (Kotagal, 2000; Bleasel et al., 1997).
The combination of ipsilateral automatisms and contralateral dystonic posturing is highly characteristic of temporal lobe seizures (Kotagal, 1999; Wieser et al., 1993), and it has a good lateralizing value. Unilateral automatisms do not have a lateralizing value on their own (Kotagal, 2000; Bleasel et al., 1997).
Secondary generalization of complex partial seizures may occur; this is more common in seizures of frontal origin than in those originating in the temporal lobe, but estimates have indicated that it occurs in up to 60% of temporal lobe seizures (Kotagal, 2000). Automatisms can occur during or following generalized seizures with similar characteristics to those seen during focal seizures.
Diagnosis of Focal Seizures with Alteration of Consciousness
Difficulties in diagnosis are apt to arise in cases in which only sensory phenomena or automatisms occur because these may be difficult to distinguish from nonepileptic behavioral aberrations, especially in small children who are unable to report subjective symptoms. In the authors’ experience, focal seizures with alteration of consciousness in children are of brief duration, and they feature stereotypic and usually relatively simple manifestations. They may be overlooked, but they are rarely mistaken for nonepileptic phenomena. More complex psychic and affective manifestations are less common, and many of them are not epileptic in nature, even though they often arouse a suspicion of limbic seizures.
The following three problems of diagnosis should be considered: (a) nonepileptic, nonictal manifestations; (b) nonepileptic, ictal manifestations; and (c) other epileptic manifestations. The first category includes such phenomena as episodes of depersonalization; derealization; feelings of déjà vu or strangeness; hallucinations or illusions; or amnesic phenomena, such as flashbacks. Although all may be part of a focal seizure, they may be seen in patients who are not suffering from epilepsy (especially in adolescents) as a result of the use of certain drugs, such as cannabis derivatives or hallucinogenic agents. Similar symptoms are a hallmark of psychosis, and these may be difficult to distinguish from true epileptic events, with which they may occasionally be associated. More often, similar but vaguely described symptoms are reported by children or adolescents to attract the attention of adults or their peers. In all such patients, the duration of the events is longer than in epileptic seizures, and overt phenomena such as alimentary or simple motor automatisms are lacking, which should arouse suspicion. The common fugue of adolescents is rarely interpreted erroneously as epileptic. Occasional cases of “hysterical” fugue with amnesia have been recorded, and they very seldom raise a diagnostic problem with “complex partial” status (Gastaut et al., 1956).
A number of ictal nonepileptic events are often mistaken for epileptic seizures (see Chapter 21). Migrainous attacks, especially those accompanied by visual illusions, often raise such a problem. This is particularly the case with micropsia, often occurring in association with somatosensory illusions, which is much more commonly a manifestation of migraine than of epilepsy (the “Alice in Wonderland syndrome”) (Breningstall, 1985). In addition, confusional states occurring with migraine are at times interpreted as seizures with alteration of consciousness, although their duration is much longer (Gascon and Barlow, 1970). Recurrent abdominal pain with or without vomiting is seldom the only manifestation of focal seizures. However, ictal vomiting, with prolonged alteration of consciousness and often version, is a common feature of the early onset idiopathic childhood epilepsy with occipital paroxysms (Ferrie et al., 1997; Guerrini et al., 1994, 1997a; Panayiotopoulos, 1989b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






