31
CHAPTER
![]()
Epilepsy Surgery
Edgar Perez and Gerald Grant
More than 2 million people in the United States have epilepsy, and 400,000 to 600,000 of them have seizures that cannot be controlled by antiepileptic drugs (AEDs) (1). A series on epilepsy management has shown a decreasing rate of seizure freedom with increasing AEDs. Approximately 47% of patients became seizure free on one AED, 13% on a second AED, and 1% on a third monotherapy. Only 3% were controlled with two AEDs and none with three (2). Epilepsy surgery is indicated for patients whose seizures are refractory to medication, defined often by two failed treatments of first-line medication and one combination therapy when used at therapeutic levels over 1 to 2 years. The goals of surgery are threefold: seizure control, minimal side effects from surgery, and improved quality of life. Unfortunately, unless the patient is seizure-free, the quality-of-life measures do not significantly improve. In general, surgical options include focal resection of epileptogenic cortex or disconnection of the epileptogenic cortex or network. Notably, only complete resection of the epileptogenic focus or lesion offers a possible cure.
PREVALENCE OF SURGERY
In 1990, there were approximately 100,000 to 300,000 potential candidates for epilepsy surgery in the United States, yet surveys show that only 1,500 procedures were performed (3). Despite improvements in neurosurgical techniques and reports on its safety and efficacy, physicians and patients continue to misperceive surgical intervention as a last resort when, in reality, surgical therapeutic intervention has offered the best chance of cure. Examples of surgically remediable syndromes include:
![]() Mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS)
Mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS)
![]() Focal epilepsy caused by discrete structural lesions that can be resected without introducing additional neurologic deficits
Focal epilepsy caused by discrete structural lesions that can be resected without introducing additional neurologic deficits
![]() Catastrophic unilateral or secondary generalized epilepsies of infants and young children that result from disturbances confined to one hemisphere, such as hemi-megalencephaly, Sturge-Weber syndrome, Rasmussen encephalitis
Catastrophic unilateral or secondary generalized epilepsies of infants and young children that result from disturbances confined to one hemisphere, such as hemi-megalencephaly, Sturge-Weber syndrome, Rasmussen encephalitis
![]() Relatively large but unilateral developmental abnormalities, such as cortical dysplasias and porencephalic cysts.
Relatively large but unilateral developmental abnormalities, such as cortical dysplasias and porencephalic cysts.
These conditions can be treated with anteromesial temporal lobectomy, localized cortical resection, hemispherectomy, hemispherotomy, or multilobar resection. The best results with respect to subsequent seizures, psychosocial readjustment, and quality of life are attained when surgery is performed as soon as it can be established that high-dose first-line AEDs as monotherapy have failed.
SURGICAL EVALUATION PROCESS
The selection of candidates for epilepsy surgery has been discussed elsewhere in this textbook. Briefly, the following patient workup is usually done with each test providing a piece of information to assess for the possibility of surgical resection.
1. Medical history, including seizure semiology (type and frequency), antiepileptic drug history, daily functions and activities, quality-of-life assessment, and mood and behavioral assessment.
2. Comprehensive physical examination.
3. Brain MRI to investigate cerebral lesions and possible resectable lesions.
4. Video EEG (vEEG) monitoring to confirm the seizure type(s).
5. Functional MRI (fMRI) or magnetoencephalography (MEG) to determine language lateralization for temporal lobe cases.
6. Wada test (also known as an intracarotid amobarbital procedure, IAP) to predict memory dysfunction following a mesial temporal resection.
7. Ictal and interictal SPECT.
8. PET.
9. Neuropsychological evaluation.
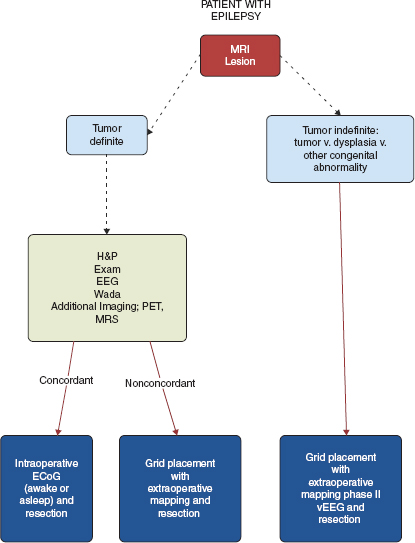
Patients may require staged grid placement for language/motor mapping versus a single-stage intraoperative mapping session followed by surgical resection. See Figure 31.1 for a suggested algorithm of patient evaluation.
FIGURE 31.1 Suggested algorithm for evaluation of a patient with refractory epilepsy and lesion on MRI.
![]()
Presurgical Evaluation: MRI
Only MRI can demonstrate anatomic alterations associated with epilepsy, such as tumor, dysplasia, mesial temporal lobe atrophy or sclerosis, and gliosis. By EEG standard, MRI has a high specificity (78%–95%) and a low sensitivity (43%–55%) for epilepsy (4) (Tables 31.1–31.2). Though structural abnormalities are highly correlated with the epileptogenic focus, they are not synonymous. As such, MRI cannot be used alone to define an epileptogenic process, and is commonly used in conjunction with EEG for assessment.
Overall, MRI can be very helpful in the diagnostic workup of a patient with severe intractable epilepsy. The most common pathological substrate for temporal lobe epilepsy requiring surgery is mesial temporal sclerosis (MTS). However, moderate hippocampal atrophy and signal change can be missed. Conversely, extreme hippocampal changes, especially on T2 and fluid-attenuated inversion recovery (FLAIR), may be erroneously called tumors when in reality they might represent severe sclerosis. It is important to keep this in mind when evaluating patients.
Presurgical Evaluation: Wada Test
The Wada test is often used in presurgical patient evaluation to determine cerebral dominance for language and memory lateralization. Its use is controversial, but the goal is to reduce severe postoperative memory loss in patients who will undergo a unilateral resection of a temporal lobe. Specifics on Wada testing are discussed in Chapter 22. Briefly, amobarbital is introduced into the internal carotid arteries and injected into one hemisphere at a time. There is no universal, standardized memory test procedure for the Wada test, and the task assessments as well as the techniques of injection vary across institutions. Having the patient name objects that are shown can assess language skills. Asking the patient to name as many objects as they can remember can assess memory. It is expected that the test will predict how well the patient’s memory will function after a resection. Ideally, memory should not be impaired when the hemisphere of planned surgery is injected, thereby confirming adequate memory on the contralateral side. A score within two standard deviations of normal verbal memory ensures that the hippocampus can be safely resected without significant deficits.
TABLE 31.1 Comparative Specifi city and Sensitivity of Neuroimaging Procedures in Epilepsy (by EEG Standard)
Individual assessment of each surgical patient is recommended when deciding who should undergo Wada testing. At many clinical sites, Wada testing is being eliminated in cases in which the fMRI are highly lateralized. fMRI has been found to be in agreement with the Wada test in 91.3% of patients (5), and has the benefit of being noninvasive and providing within-hemisphere regional localization of function. However, care must be taken in using fMRI for language lateralization and sources of error must be considered. If the results from fMRI lateralization and neuropsychological testing are equivocal, as can be common in temporal lobe epilepsy, Wada testing is used to resolve the issue. Refer to Figure 31.2 for a suggested algorithm for determination of Wada testing. Though not all patients will need a Wada test, it is incorrect to always assume that a patient who is left-hemisphere dominant with right medial temporal lobe epilepsy will not need it. Wada testing will depend on neuropsychological testing of the contralateral lobe. In this scenario, intraarterial studies would be indicated if neuropsychological testing showed poor verbal memory in order to ascertain that the patient has capacity for memory support if the dominant hippocampus is resected. Wada testing could be deferred if the patient has asymmetric memory, eg, poor memory on the ipsilateral side and spared memory on the contralateral side, and is noted to be clearly dominant on fMRI.
TABLE 31.2 Comparative Specificity and Sensitivity of Neuroimaging Procedures in Temporal Lobe Epilepsy (by Pathology Standard)
| SENSITIVITY | SPECIFICITY |
PET | 81% | 22% |
Interictal SPECT | 70% | 36% |
Ictal Spect | 93% | 13% |
MRI | 69% | 68% |
Source: From Ref. (5). Arora J, Pugh K, Westerveld M, et al. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225–2241.
In general, memory impairment implicating the role of the contralateral hippocampus in cases with an apparently unilateral mesial temporal focus is a contraindication for surgical treatment. A patient with intractable epilepsy and temporal onset, and with a normal ipsilateral hippocampus and good memory is the patient at higher risk for memory decline following an amygdalohippocampectomy.
Drawbacks of the Wada test include: (a) a morbidity as high as 5% of causing a stroke due to the angiogram (6), (b) patient discomfort—many patients dislike the paralysis and speech arrest induced by the procedure; (c) an inability to localize speech or other primary language functions, and (d) a high cost.
Decision Making and Indications of Substrate-Directed Surgery
Patients may undergo resection if the epileptogenic focus is concordant with other data (eg: imaging, vEEG results, PET, SPECT, neuropsychological testing, and MEG) and if that region can be safely removed without causing significant deficits. If the seizure focus overlaps with eloquent cortex, then multiple subpial transections can be considered. In cases showing generalized forms of epilepsy onset, surgery is commonly not recommended. However, in certain scenarios, such as in a child with drop attacks or atonic seizures, a corpus callosotomy may be beneficial, as will be discussed. All cases should be evaluated individually for risks and benefits, and be discussed at multidisciplinary epilepsy management team meetings.
Substrate-directed surgery is indicated with concordant MRI substrate and EEG. Refer to Figure 31.3 for a suggested algorithm for substrate-directed surgery. If the medial temporal lobe is discovered to be the site of seizure focus, and it is in the nondominant hemisphere, anteromedial temporal resection (AMTR) is indicated. If the seizure focus is in the dominant hemisphere, AMTR would still be indicated if the patient passed their Wada test and it was felt that the patient’s memory would be supported by the contralateral hippocampus.
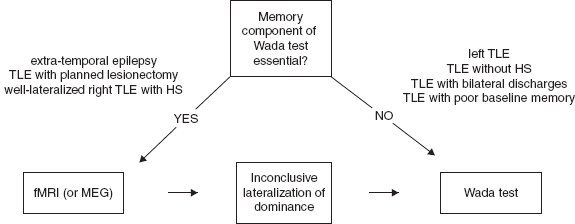
FIGURE 31.2 Suggested algorithm for presurgical assessment of language dominance.
![]()
Surgical resection of a neoplastic substrate is dependent on location. If the neoplasm is within the medial temporal lobe, the patient may require medial resection, including the hippocampus, for best seizure control. If it is a lateral tumor and the hippocampus looks normal, the hippocampus may be spared unless the workup reveals that the seizures originate there. If the tumor is found in any other lobar positions, resection to the edge of the lesion or as defined by the EEG localization of the epileptogenic zone is indicated. The same paradigm holds true for cavernous hemangiomas, which are one of the most common forms of vascular lesions to cause medial lobe temporal epilepsy. Invasive ictal recordings with a grid in place may be helpful to safely resect arteriovenous malformations (AVMs) in eloquent cortex or in a patient with an AVM and refractory epilepsy.
In patients with dual pathology, eg, the presence of an extrahippocampal lesion plus hippocampal atrophy, resection of both the lesion and the medial structures is reported to be associated with improved outcomes than either alone (7). In a study on surgical outcomes for dual pathology epilepsy, lesionectomy plus mesial temporal resection resulted in complete freedom from seizures in 73% patients, while only 20% patients who had mesial temporal resection alone and 13% who had a lesionectomy alone were seizure free (7). An intracranial study to verify dual pathology, especially in the case of the dominant temporal lobe, is important in order to preserve functionally intact medial structures.
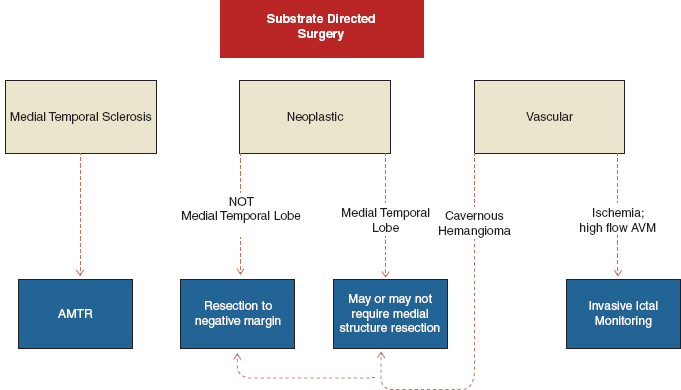
FIGURE 31.3 Suggested algorithm for substrate-directed surgery.
![]()
INTRACRANIAL ELECTRODE STUDIES
Intracranial electrodes help lateralize the seizure focus, and also help in brain mapping of eloquent areas. There are four main clinical scenarios that are likely to require an intracranial study.
1. Patients who harbor dual pathology, nonlesional epilepsy, extratemporal epilepsy, and lateral temporal lobe epilepsy will frequently require intracranial electrodes to map the epileptogenic focus using electrocorticography (ECoG) and the adjacent functional cortex using cortical stimulation (9).
2. Patients with bilateral independent temporal lobe spikes or bilateral MTS will require intracranial electrodes to lateralize the dominant epileptogenic temporal lobe and improve the success for postoperative seizure freedom (10). Once laterality of seizure onset has been determined, the patient may then be a candidate for placement of grid and additional strip electrodes. However, bilateral craniotomies for grid placement are not routinely performed due to the surgical morbidity.
3. Patients whose workup is discordant or inconclusive may require intracranial electrodes for further monitoring and assessment (11).
4. Young patients who are unable to tolerate awake cortical mapping for resection of their tumor may require intracranial electrodes for extraoperative mapping.
Intracranial Electrode Placement
If an intracranial study is indicated, the placement of intracranial electrodes is guided by the results of Phase-1 presurgical evaluation, including scalp vEEG recording, MRI, and other imaging. Electrodes are inserted through 5-mm burr holes placed both frontally and temporally on both sides of the brain. Most often, anterolateral and posterolateral temporal electrodes are inserted in addition to a subtemporal electrode. Preference of strip electrodes and depth electrodes depends on the epilepsy center and experience of the team. (See the following for advantages and disadvantages of the different electrode types.) A frontal burr hole may be considered if there is a suggestion of orbitofrontal involvement or rapid spread to the frontal lobe. The frontal burr hole, made just behind the hairline and parasagittal, is used to place three frontal strips: lateral, anterior subfrontal, and interhemispheric. For extraoperative ECoG, the electrodes are sutured along their border to the edges of the dura to avoid displacement during the monitoring period, and the dura is then closed in a “water-tight” fashion.
Strip Electrodes
A strip electrode is a single linear array of disk electrode contacts. See Figure 31.4A
Advantages. Strip electrodes do not penetrate the cortex and are surface based. Multiple strip electrodes can be placed through a burr hole, requiring less operative time. They can be used for extraoperative cortical stimulation to map out functional areas of the brain.
Maneuvers using strip electrodes can facilitate recording from specific regions: (a) a strip advanced around the temporal pole and underneath the lesser wing of the sphenoid bone will follow the medial temporal lobe contour, and invariably end up along the medial basal temporal lobe surface, allowing adequate coverage of the parahippocampal gyrus along its long axis extending posterior to the level of the collicular plate (12); (b) a strip advanced along the posterior temporal lobe in the posteromedial direction is guided by the surrounding dural structures including tentorium, and invariably ends up at the occipital interhemispheric space. This strip allows for medial occipital recording or cortical stimulation of the visual cortices. In addition, this strip samples the basal surface of the posterior temporal and occipital lobes (13).
Disadvantages. Strip electrodes cannot record deep brain structures. In addition, placement can be unpredictable and inaccurate by virtue of the insertion technique and experience of the surgeon (14).
Depth Electrodes
A depth electrode is a thin wire that can be placed in deep brain structures.
Advantages. Depth electrodes help to determine laterality. They can record from deep brain structures. Three depth electrodes are often placed from lateral to mesial to record from the amygdala, anterior, and posterior hippocampus.
Disadvantages. There is a 2% to 3% risk of an intracerebral hemorrhage (potentially due to burr hole placement with parietal–occipital burr hole trajectories avoiding the multiple convolutions of the temporal lobe where major branches of the middle cerebral artery (MCA) can be found, and minimizing bleeds) (15). Only one depth electrode can be placed per twist drill hole. In addition, depths are associated with longer operative times and increased cost due to the use of stereotactic equipment for placement. Note: histopathology of resected tissue reveals needle track evidence with gliosis and inflammation from depth electrode placement. However, this pathology is believed to be clinically insignificant, and there is no published evidence supporting permanent cognitive dysfunction.
Grid Electrodes
A grid electrode is an array of parallel rows of disk electrode contacts (see Figure 31.4B).
Advantages. Grids have the ability to localize seizure focus and conduct language and motor mapping. Grids give ample coverage of language sites as well as face and hand motor areas.
Disadvantages. Grids necessitate a large craniotomy and increase the risk of a subdural hematoma (15).
Electrocorticography
Subdural recordings are associated with a higher sensitivity for detecting epileptiform discharges than scalp EEG. There are two types of intracranial ECoG, intraoperative ECoG and extraoperative ECoG. For intraoperative ECoG, the electrodes are placed on the brain during a surgical procedure to remove a lesion causing epilepsy, and are removed at the end of the surgery. This procedure is only done in the operating room. For extraoperative ECoG, the electrodes are placed in or over the brain in areas suspected to be epileptogenic foci, and are left in place. The patient is then brought back to the ward where recordings are made to capture seizures. The electrodes are removed at a later date.
Intraoperative Electrocorticography
Intraoperative ECoG offers flexible placement of recording and stimulating electrodes, the ability to directly stimulate regions of the brain to determine which parts to spare, and the ability to assess the presence or absence of interictal epileptiform activity immediately pre- and postresection. However, intraoperative ECoG has limitations, including limited sampling time, exclusive interictal spikes and sharp wave recordings, and a lack of seizure (ictal) capture. It is also impossible to distinguish primary epileptiform discharges from secondarily propagated discharges arising at a distant epileptogenic site. In addition, background activity and epileptiform discharges may be altered by the anaesthetics and/or narcotic analgesics required by the surgery itself.
While the use of intraoperative ECoG in resection surgery has been an accepted clinical practice, recent studies have shown that the usefulness of this technique varies on the patient’s type of epilepsy. Studies suggest a critical value of intraoperative ECoG in lesion-related frontal lobe epilepsy (17), lesion-related temporal lobe epilepsy (18,19), subpial transections, and cortical dysplasias (8,20). However, it has been found impractical in the standard resection of MTLE with MRI evidence of MTS. In a series involving resections with intraoperative ECoG, there was no correlation between residual spikes on pre- and postresection ECoG and outcome. These findings do not support the role of intraoperative ECoG in guiding standard mesial temporal lobe resections (18). Finally, the added impact of interictal spike frequency measures was modest, if any, in cases with available ictal ECoG and neuroimaging (21). However, although chasing spikes has not been proven to improve outcome following surgery, it may still be done for prognostic reasons since residual spikes may be a poor prognostic feature.
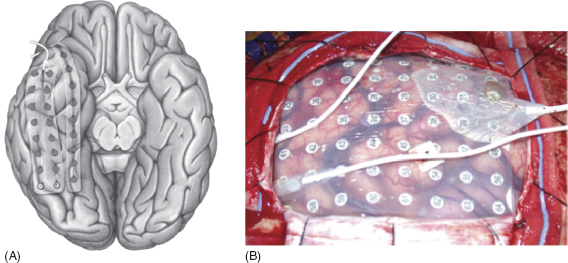
FIGURE 31.4 (A) Strip and (B) grid electrode.
Source: From Refs. (13)A; and (16)B.
![]()
Extraoperative Electrocorticography
In the absence of an observable lesion to direct the surgical intervention, centers often rely on more extensive ECoG or on extraoperative ECoG monitoring to map the ictal onset prior to cortical resection. The seizure onset zone can be determined only after a seizure occurs; thus, some patients may have to undergo monitoring for more than a week until they experience a seizure. A typical intracranial monitoring study lasts 4 to 10 days postoperatively. Benefits and risks must be assessed in lengthier monitoring periods since a recording period of more than 10 days may increase the risk of complications, including infection (22), and increase the total medical cost.
Ictal ECoG recordings during extraoperative monitoring are visually reviewed daily to determine the electrode contacts where the seizures begin. Seizure onset is defined as a sustained rhythmic change on ECoG accompanied by subsequent clinically typical seizure activity, not explained by state changes, and clearly distinguished from background and interictal activity. Unfortunately, the placement of electrodes alters the normal electrical environment of the brain, potentially leading to temporary cessation of seizures or alternation of a patient’s habitual seizure semiology. As a result, it is important to confirm that the recorded seizures during an intracranial study are similar to the patient’s habitual seizures and even consider reducing the antiepileptic medication prior to surgery in a patient with rare intractable seizures.
TECHNIQUES AND TYPES OF SURGICAL RESECTION
The basic technique for resection of epileptogenic tissue involves coagulating the surface or pial vessels, sharply incising the pial membrane covering the cortex, and using fine suction or bipolar cautery to dissect through the gray matter tissue down to white matter. Care is taken to not resect more than needed to preserve the white matter tracts as long as they are not involved in the functional epileptic network. There are several types of surgical procedures that can be performed to treat epilepsy. These include temporal lobectomies, lesionectomies (resection of MRI evident abnormal brain tissue), corpus callosotomies, hemispherectomies, and multiple subpial transections (MST).
Temporal Lobectomy
Temporal lobectomy is the most common surgical procedure performed for medically refractory epilepsy. A standard lobectomy consists of the removal of the entire temporal lobe, including the mesial structures; however, this is rare today. Currently, temporal lobectomies involve a more limited resection in order to try to conserve as much neuropsychological function as possible. There are two main types of temporal lobe surgeries for epilepsy: AMTR and selective amygdalohippocampectomy (SAH), of which there are three main surgical approaches that will be discussed.
Standard Anterior Temporal Lobectomy
In a standard anterior temporal lobectomy (ATL), resection of the lateral temporal structures allows for visualization of the mesial structures and removal of the hippocampus.
Briefly, the patient is supine with the ipsilateral shoulder elevated. The head is lateral with the zygoma at a 10-degree angle from the horizontal plane. A craniotomy is performed on the frontal bone posterior to the pterion. A posterior cortical incision at the lateral temporal gyri begins approximately 5.5 cm from the temporal tip on the nondominant hemisphere and 4.5 cm from the temporal tip on the dominant side at the level of the middle temporal gyrus. Posteriorly and inferiorly, the dissection continues down to the level of the collateral sulcus. The dissection then continues anteriorly to the anterior tip of the temporal lobe and laterally to the floor of the middle fossa. The anterolateral temporal lobe is removed. The white matter is dissected along an imaginary plane to identify the temporal horn of the lateral ventricle, which is usually at the intersection of the plane of the collateral sulcus and superior temporal sulcus. The parrahippocampus that includes the uncus is then resected subpially followed by an amygdalohippocampectomy.
Anteromedial Temporal Resection
In the 1980s, a modified approach, AMTR, was introduced for temporal lobectomies for patients with medial temporal ictal onset, such that all of the medial structures are removed via a limited temporal pole resection, sparing the superior temporal gyrus.
The resection involves a 3- to 3.5-cm neocortical resection of the pole, including the middle and inferior temporal gyrus, as access to the temporal horn, facilitating the resection of the important structures that provide the triad for epileptogenesis in the medial temporal lobe, namely the entorhinal cortex, the amygdala, and the hippocampus (Figure 31.5).
Briefly, the patient is positioned supine with the head extended 50 degrees backwards towards the ipsilateral shoulder; this head extension is critical to throw the hippocampus into a perfectly aligned view giving microscopic exposure of the hippocampal tail. A curvilinear incision is made in order to expose a sizeable portion of the temporal lobe: the posterior skin incision is at the point of the mastoid vertex and the anterior curve is at the anterior hairline. The skin and temporalis muscle are elevated as a single flap and reflected anteriorly. A standard craniotomy is done, exposing the posterior temporal neocortex. This exposure allows for lateral mobilization of the temporal neocortex in order to visualize the posterior tail of the hippocampus later in the procedure. The inferior temporal pole resection involves 3.5 cm of middle and inferior temporal gyrus. The first corticotomy is conducted along the superior temporal sulcus by coagulating pia and cutting across. Preservation of the vein of Labbé is important. In addition, the superior temporal gyrus is not violated. The lateral temporal neocortex is removed from the superior temporal sulcus using arachnoid of fusiform gyrus and middle fossa protuberance as landmarks. This technique helps the surgeon keep out of the temporal stem, which has no landmarks. The center of the middle temporal gyrus is then used as a landmark to open the ventricle lateral to the hippocampus without injuring it. A small part of the ventricle is opened. A white matter dissection is done along the occipital temporal fasciculus, mobilizing the temporal lobe laterally to see around the hippocampus.
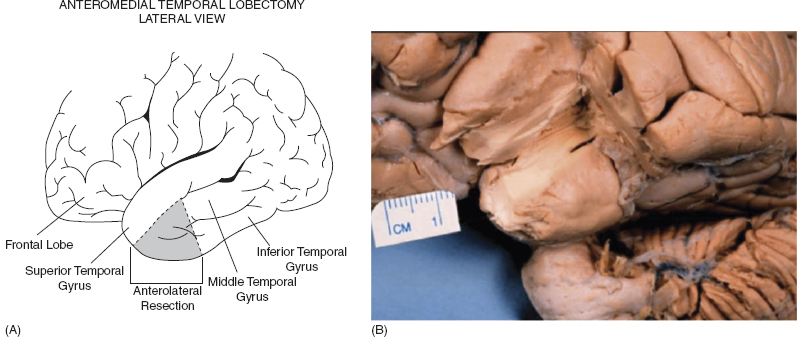
FIGURE 31.5 AMTR.
Source: From Ref. (23). Spencer D. Medial Tempral Lobe Epilepsy: Evaluation. In Cohen-Gadol A, ed. ANA: Grand Rounds Video Conference. 2013.
![]()
An imaginary line drawn between the turn of the MCA, or approximately the MCA bifurcation, and the anterior edge of the choroid plexus in the temporal horn, the inferior choroidal point, forms a reliable landmark for defining the superior extent of resection during removal of the amygdala (24). The amygdala can then be removed in a selective manner. Once the amygdala is removed, the third nerve is found underneath the arachnoid to ensure that the amygdalectomy is complete. Afterward, the choroid plexus is disconnected from the hippocampus and care is taken to avoid injuring the anterior choroidal artery at the choroidal point in the ventricle. Then, the hippocampus and parahippoccampus are undermined from the arachnoid over the basal cisterns.
Selective Amygdalohippocampectomy
In SAH, the neurosurgeon resects the medial temporal structures, including the amygdala and the hippocampus, but leaves the lateral temporal lobe intact. The rationale for SAH has been that it should provide equivalent seizure control because the mesial structures, the presumed source of the seizures, are removed with limited damage of the lateral temporal neocortex and the underlying white matter, possibly better preserving neurocognitive functions. This is becoming a common temporal resection performed at many centers.
Niemeyer proposed the SAH in 1958 using a transcortical incision through the middle temporal gyrus (Figure 31.6). In 1985, Yasargil and Wieser advocated an SAH with a different approach, the transsylvian approach through the deep Sylvian fissure. Hori (25), Park (26), and Little (27) have also described variations of the SAH using a subtemporal corridor. In this approach, the amygdala and hippocampus are removed through an opening in either the fusiform or parahippocampal gyrus.
These approaches have been shown to result in similar favorable seizure-freedom outcomes. Better cognitive outcomes have been suggested with the transsylvian SAH since there is less collateral neocortical damage. In general, the main complications in SAH are vascular injuries.
Transcortical approach. This approach involves a craniotomy centered on the projection of the middle temporal gyrus, followed by a longitudinal cortical incision through the middle temporal gyrus. An alternative approach is through the anterior superior temporal gyrus. The surgeon may use neuronavigation devices to direct the dissection to the temporal horn of the lateral ventricle. Once in the ventricle, the choroid plexus with the choroid point is identified. Subsequently, the uncus is emptied, and the amygdala and hippocampus are disconnected from the surrounding structures and removed subpially.
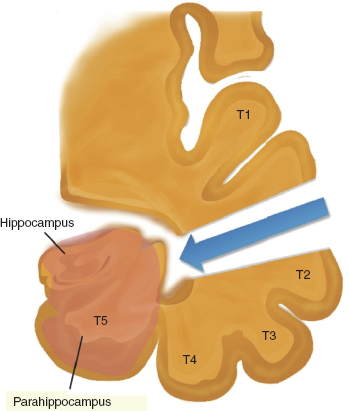
FIGURE 31.6 Transcortical approach.
Source: From Ref. (28). Al-Otaibi F, Baeesa SS, Parrent AG, et al. Surgical techniques for the treatment of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:374848.
![]()
Disadvantages: a portion of the lateral temporal neocortex must be dissected.
Transsylvian (Yasargil) approach. The surgeon performs a pterional craniotomy of approximately 5 cm in diameter followed by microsurgical dissection of the sylvian fissure (2.5–3 cm). The surgeon reaches the temporal horn of the lateral ventricle via the inferior limiting sulcus. Once in the ventricle, the surgeon resects the mesial structures as described earlier.
Advantages: This approach avoids injury to the temporal neocortex and underlying white matter, which are traversed in the transcortical approach. It also allows en bloc resections. The transsylvian approach has been suggested to transect fiber pathways important for seizure propagation, leading to better seizure outcomes; but this has not been confirmed.
Disadvantages: The transsylvian approach is technically difficult due to a limited (2–2.5 cm) cortical incision and the presence of major blood vessels in the sylvian fissure. There is a greater chance of injuring the M1 portion of MCA within the sylvian fissure. There is also inevitably injury to the superior temporal, inferior temporal, and fusiform gyri (29). This approach results in transection of the temporal stem, usually by approximately 20% (30).
Subtemporal approach. This approach involves opening the temporal horn from the basal surface of the temporal lobe, thereby sparing the lateral neocortex and temporal stem, which are affected in a traditional transsylvian approach.
Advantages: The subtemporal approach preserves functional temporal lobe tissue in the superior, middle, and inferior temporal gyri. It does not disrupt frontotemporal white matter pathways that reside within the temporal stem. In addition, the visual fibers near the roof of the temporal horn are spared, avoiding visual field defects. It might produce fewer neuropsychological sequelae, though data are insufficient to make this claim (31,32).
Disadvantages: It may require excessive retraction of the temporal lobe and removal of the zygomatic process. It may result in injury to the vein of Labbé.
Selective Amygdalohippocampectomy Versus Anterior Temporal Lobe Resection
The efficacy of SAH versus ATL remains controversial. Refer to Figure 31.7 for a visual comparison of anterior temporal lobectomy and selective amygdalohippocampectomy. A literature review concluded that patients who underwent an SAH have similar seizure outcomes and better cognitive outcomes than those who underwent an ATL resection (33). Since SAH is also less invasive, this procedure has become popular in most centers. However, a 2013 literature review and meta-analysis of data showed a modest but statistical difference between ATL and SAH, with 8% more patients with MTLE becoming seizure free after ATL (34). Given the limited quality of evidence, neither SAH nor ATL resections can officially be recommended over the other option as a standard or guideline for the surgical management of TLE.
Anatomic Considerations in Temporal Lobectomy
Anatomical structures/landmarks considered in a temporal lobectomy include Wernicke’s area (if dominant hemisphere), optic radiations (if non-dominant hemisphere), the Sylvian fissure, and the incisura.
In a temporal lobectomy of the dominant hemisphere, the surgeon is careful to preserve Wernicke’s speech area. Classically, dominant hemisphere anterior temporal cortex in the middle and inferior temporal gyri are not considered essential for language. There are visual and auditory naming areas however in the middle temporal gyrus on the dominant side. The line of the central fissure or the vein of Labbé, located 4 to 5 cm from the temporal tip, has been proposed as the posterior limit of the “safe” zone. It is usually safe to resect up to 4 to 5 cm from the temporal tip.
If the temporal lobectomy is performed on the nondominant hemisphere, the surgeon is most concerned with the optic radiations, which contain all the visual fibers of the opposite half of the visual field. The optic radiations course anteriorly over the roof of the inferior horn, then backward, forming the Meyer’s loop. Transections of fibers in Meyer’s loop can lead to superior homonymous quadrantanopia, which may be acceptable and does not disturb the patient in daily life.
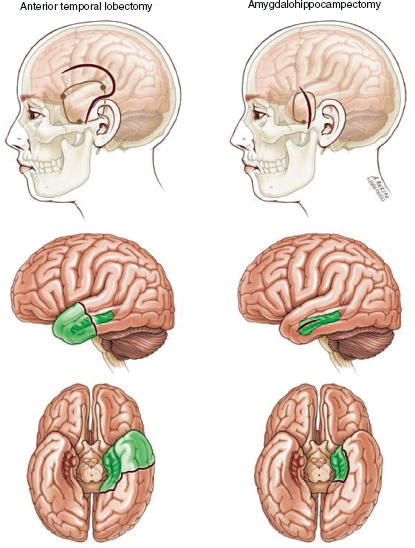
FIGURE 31.7 Comparison of anterior temporal lobectomy and selective amygdalohippocampectomy.
Source: From Ref. (35). Spencer D, Burchiel K. Selective amygdalohippocampectomy. Epilepsy Res Treat. 2012;2012:382095.
![]()
Corpus Callosotomy
The corpus callosum is the most important pathway for rapid interhemispheric spread of epileptic activity (36). The simple premise of a corpus callosotomy is that severing the connections between the hemispheres will stop the spread of seizures. In addition, the theory is that if neurons in both hemispheres are required for seizure onset, then severing them might also help reduce seizure frequency. Importantly, the goal of a corpus callosotomy is palliative, not seizure freedom. This surgery is indicated for patients who are medically refractory and who are not candidates for a focal resective surgery and include: (a) patients with drop attacks that lead to injuries (b) patients with generalized seizures involving unilateral hemisphere damage, and (c) some patients with generalized seizures without an identifiable, resectable focus.
Surgical Technique
A bifrontal craniotomy is performed, and via an interhemispheric approach an anterior 2/3 callosotomy or full callosotomy is generally performed. Image guidance can be helpful to delineate the extent of the callosotomy. The two pericallosal arteries lie directly over the callosum and should be protected and avoided (Figure 31.8).
Outcomes
The chance of seizure freedom after a corpus callosotomy is only 10%. So this procedure is primarily for patients who might benefit from reduced risk of injuries from seizure reduction, such as those with drop attacks who fall a lot and need to be in a helmet for safety. Therefore, the procedure is only meant to be palliative. Even drop attacks are not completely eliminated. A meta-analysis shows that in the long term (>5 years), only 35% of patients are drop-attack free (38).
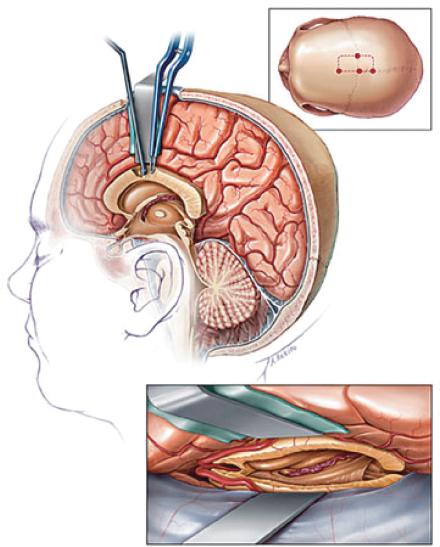
FIGURE 31.8 Callosotomy approach.
Source: From Ref. (37). Rahimi SY, Park YD, Witcher MR, et al. Corpus callosotomy for treatment of pediatric epilepsy in the modern era. Pediatr Neurosurg. 2007;43(3):202–208.
![]()
Based on outcome studies, the benefits of a corpus callosotomy are as follows: seizure reduction, reduced risk of injury, improved overall daily function, and family satisfaction. Subsequent quality of life is difficult to assess because patients often present with severe mental retardation or developmental delay, and family satisfaction has been increasingly used as a surrogate marker. Notably, corpus callosotomy is associated with low morbidity.
The outcome for drop attacks is more likely to be favorable if the patient has clinical, radiologic, or EEG evidence of a unilateral lesion. True generalized slow spike and wave activity, characteristic of Lennox-Gastaut syndrome, is also associated with a favorable outcome. The presence of independent bilateral spikes on preoperative EEG and severe mental retardation are associated with unfavorable outcomes, especially for drop attacks.
Anterior Corpus Callosotomy Versus Complete Corpus Callosotomy
In an anterior 2/3 callosotomy, the splenium is spared to preserve sufficient fibers for interhemispheric transfer of some perceptual information and to diminish the risk of disconnection syndrome. However, the chance of a callosotomy exhibiting a significant impact on the quality of life of patients tends to be proportional to the extent of the disconnection (39). The complete arrest of drop attacks (atonic and tonic seizures) may be observed in up to 91% of pediatric cases by total callosotomy, but only in 67% by partial section. In terms of neurologic complications, the frequency is nearly equal in partial and complete resections; however, complication severity is comparatively less in partial resections (40). Case series have shown a stronger association of disconnection syndrome after complete callosotomy versus partial (40). A literature review reported split-brain syndrome in 33% of patients after total or near-total callosotomy, with 30% of the cases transient and 3% persistent (41).
Complications
Complications of corpus callosotomy include disconnection syndrome, language impairment and transient aphasia, transient akinetic state, memory deficits, mild neuropsychological impairment, new types of seizures, aseptic meningitis/ventriculitis after exposure of the third ventricle, transient neurological deficits, permanent neurological deficits, and death.
Disconnection Syndromes
Disconnection syndromes can occur following complete corpus collosotomies. These syndromes are characterized by the absence of interhemispheric relay of information from a stimulus presented unilaterally. In general, the separated hemispheres are unable to share information about stimulus identity, shape, and higher-order associations (42,43).
A severe form is the alien-hand syndrome, in which a patient’s speaking left hemisphere cannot name or identify an object held in the left nondominant hand, but the patient can select the object under guidance from his right dominant hemisphere. In clinical practice, this is rarely seen; instead, more complex neurological signs are more common: mutism, ataxia, alexia, hemineglect, gait apraxia, and urinary incontinence.
Neurological deficits depend on which fibers are disrupted at the site of the callosal lesion. Postmortem morphological studies have shown that connections of the corpus callosum are loosely organized in a rostral–caudal manner, with the frontal lobes occupying the rostral portion of the callosum and the parietal, temporal, and occipital lobes following in an anterior–posterior representation (Figure 31.9). Lesions at the isthmus of the corpus callosum, for instance, are likely to result in alien hand syndrome as information relayed to the precentral gyrus, a brain region involved in inhibitory control over involuntary motor responses, are disrupted (44). See Table 31.3 for a summary of disconnection syndromes.
Corpus Callosotomy Versus Vagus Nerve Stimulation
The advent of vagus nerve stimulation (VNS) for the palliation of severe seizure disorders has put in question the indications for callosotomies. In one study, callosotomies were found to provide a slightly better control rate (79% vs. 50% reduction of seizures by 50% or more) and similar rates of control for atonic seizures. Yet complications of callosotomies were also higher, 21% versus 8% compared with VNS (39). An advantage of VNS is that it is associated with less immediate morbidity, given that it is less invasive than a callosotomy; but, some studies report lower overall permanent morbidity rates for callosotomies. Since VNS is less invasive, it is often tried first (39), although many still believe that the outcome would be better after a corpus callostomy particularly in a patient with atonic drop attacks.
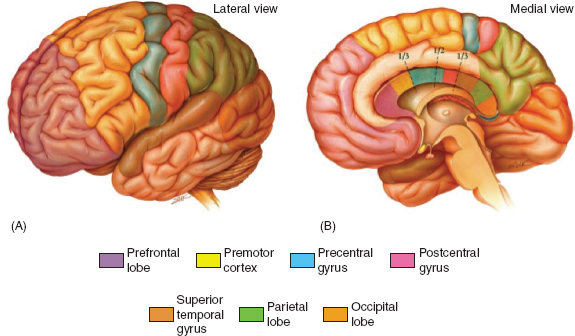
FIGURE 31.9 Topography of cerebral cortex and associated commissural fibers.
Source: From Ref. (45). Jea A, Vachhrajani S, Widjaja E, et al. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. 2008;24(6):685–692.
![]()