Lennox and Cobb (13) reported that a long disease duration correlated with an increase in the incidence of auras and wrote that “It is more accurate to speak of the recollection of aura(s) rather than of their presence.” Young children may lack the verbal capacity to describe the sensations that herald a seizure, even though their actions indicate some awareness of the impending event. Similarly, adults who deny any warning, nevertheless, may press the seizure alarm button during video–EEG monitoring but have no recollection of having done so. Some patients without auras might have had them earlier in the illness. Anecdotal experience suggests that auras may disappear as the disease progresses. The seizure either induces an amnesia so immediate that there is no memory of a warning or causes retrograde amnesia. This is supported by a study that showed that amnesia for auras depended on the severity of the seizure (18). An isolated aura is nearly always recollected when there is a unilateral EEG ictal discharge. The aura is more likely to be forgotten if the seizure becomes secondarily generalized or involves a bilateral EEG ictal discharge.
Isolated auras can persist after epilepsy surgery despite the complete cessation of complex partial (focal seizures with dyscognitive features) or secondarily generalized seizures. Isolated postoperative auras occurred in 15% to 35% (19–21) of patients after surgery for temporal lobe epilepsy and in 22% of a series after focal resective surgery unselected for location (22). Persistent auras after temporal lobe surgery may relate to incomplete removal of epileptogenic tissue in residual temporal lobe structures or neighboring areas of the operculum and insula. When epigastric auras persist after functional hemispherectomy, the insula is again implicated as it is the only cortical structure still connected on the side of surgery. Although isolated postoperative auras are often regarded as of little significance, and classified among the “seizure-free” outcomes, they may portend a reduced chance of complete seizure control (23) even if published studies do not all agree on this point, and a reduced quality of life on self-assessment (22). A small number of patients may lose their aura after temporal lobectomy even as they continue to have postoperative seizures leaving them with no warning; yet others may experience a different aura (19).
CLINICAL LOCALIZATION
An aura provides evidence of focal seizure onset and can help to localize the epileptogenic zone, provided the symptoms share a certain stereotypy and consistency even if they may vary from time to time in the same patient. Caution though is advised when interpreting the report of preceding sensation in the evaluation of a first seizure owing to the limited reliability of a single observation (24).
Patients with psychogenic nonepileptic seizures (PNES) can report a range of sensations, sometimes difficult to distinguish from that of an epileptic aura with alterations in smell, taste, vision, or tingling; but more often, the symptoms are long lasting and nonspecific, with headache, pain, and confusion prominent. Rarely, a PNES starts with an epileptic aura (25) in a patient whose epileptic seizures are well controlled. The PNES that follows an epileptic aura can be interpreted to represent a learned response or an example of psychogenic elaboration.
Current concepts on the localizing value of auras rely heavily on the pioneering studies of Penfield and Jasper (26) who correlated sensations and signs obtained through electrical stimulation of the awake patient with those of the patient’s spontaneous seizures. Subsequent studies with long-term intracranial electrodes for the recording of spontaneous seizures and extraoperative electrical brain stimulation have extended early observations (27–29).
Although an aura may help to localize the epileptogenic zone, an important point must be kept in mind. The initial sensation of an aura is related to the first functional brain area affected by the seizure that has access to consciousness, but this may not be the site of seizure origin. A seizure starting in the posterior parietal region may be initially asymptomatic until ictal activity spreads to adjacent functional areas. Spread to the postcentral gyrus may elicit a somatosensory sensation as the first warning; propagation to parietooccipital association cortex may give rise to initial visual illusions or hallucinations. Furthermore, it remains unclear whether experience of an aura is contingent on direct ictal activation of the cortical areas subserving those functions or whether an aura sensation may also be evoked by remote excitation or decoupling of inputs within a larger functional network. A sensory jacksonian march cannot be explained by other than ictal spread along the somatosensory cortex. The sometimes indistinguishable auras found in patients with hippocampal sclerosis and temporal neocortical pathology support the role of a distributed network that functionally links the limbic and neocortical structures in the temporal lobe. Cortical stimulation in extratemporal epilepsy also showed that sites at which an aura is reproduced can extend well beyond the expected functional map for those sensations (29).
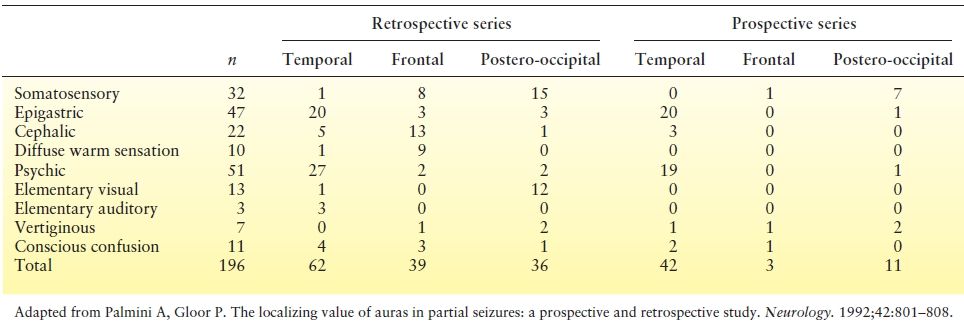
The localizing value of auras has been studied in a number of ways. Penfield and Kristiansen (30) recorded the initial seizure phenomenon in 222 patients with focal epilepsy and commented on the likely localization of different auras. Auras reported in patients with well-defined epileptogenic foci in different brain regions can be compared from different series (Table 10.2) or, better yet, prospectively (35) (Table 10.3). Data from patients (31,32) who become seizure free after localized brain resections are particularly important. Making comparisons from different series in the literature is hampered by several problems: Terminology of aura type is not uniform, auras can be grouped together in dissimilar ways, and classification rules are unclear when multiple sensations occur in the same aura. In spite of the different approaches, retrospective and prospective series yielded a similar conclusion: Auras have localizing significance. Patients with temporal lobe epilepsy have the highest incidence of epigastric, emotional, and psychic auras (31,35,36). Frontal lobe epilepsy is distinguished by frequent reports of no aura (32,35). When an aura is present in frontal lobe epilepsy, cephalic, somatosensory, and general body sensations predominate. Perirolandic epilepsy with centroparietal foci and parietal lobe epilepsy are most likely to experience somatosensory aura (33,37). Not surprisingly, occipital lobe epilepsy has the highest incidence of visual aura (34,38). No single aura sensation is necessarily restricted to a single lobe, however.
Table 10.2 Relative Incidence of Auras in Focal Epilepsies (%)
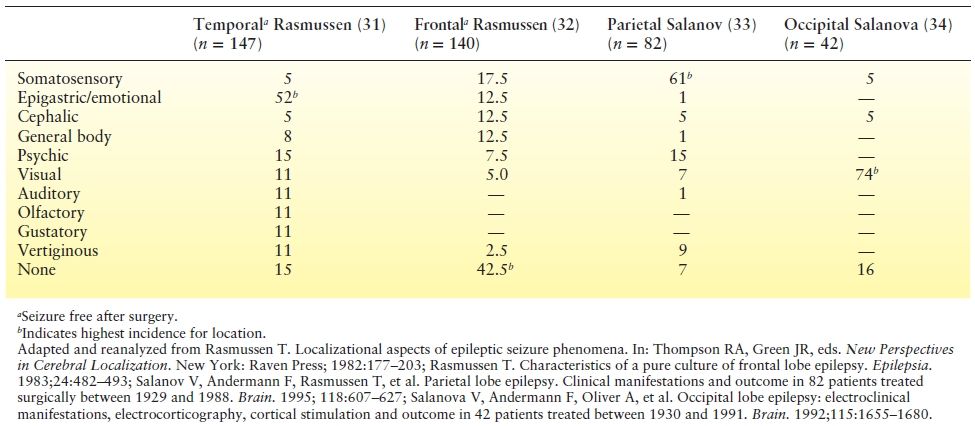
Table 10.3 Frequency of Auras in Focal Epilepsies
While most auras cannot lateralize the side of seizure onset, there are exceptions. Unilateral somatosensory aura in an extremity and lateralized visual aura are contralateral to the side of seizure onset. A lateralized auditory aura (sound in one ear) may also refer to the contralateral hemisphere. Pilomotor seizures, if unilateral, however, point to the ipsilateral hemisphere. Ictal headaches, if lateralized, are likewise ipsilateral to the site of seizure onset.
FUNCTIONAL CORRELATIONS
The EEG signature of auras depends on the recording technique. An isolated aura is a focal seizure of restricted extent and may not be apparent in scalp EEGs. In a study of temporal lobe epilepsy (39) studied by a combination of surface and depth electrodes, only 19% of auras confirmed by depth recordings had a surface ictal EEG correlate. In the same study, 10% of depth electrode recorded subclinical EEG seizures, and 86% of clinical seizures were accompanied by surface changes. An EEG that incorporates sphenoidal electrodes may have a better chance (28%) of detecting an electrographic change during auras in patients with temporal lobe epilepsy (15). The surface EEG ictal pattern is often subtle as compared with that of a complex partial seizure and may appear as low-frequency rhythmic sharp waves, sudden attenuation of the ongoing background, or abrupt cessation of ongoing interictal spikes sometimes followed by rhythmic slow waves.
Depth electrodes targeted directly at mesial limbic structures (where the majority of temporal lobe seizures originate) have been more successful in demonstrating EEG ictal activity in temporal lobe auras than were simultaneous recordings from subdural electrodes over the lateral temporal convexity (40,41). Nevertheless, even in patients with clinical seizure onset in one temporal lobe localized by depth electrodes, only about half of isolated auras showed an ictal EEG correlate (16,42). In the same patient, some auras may be associated with ictal EEG changes, whereas others show no change (43). This suggests that seizures may arise dynamically from different discrete areas within a larger epileptic zone. In neurophysiologic terms, these observations support the belief that only a very small portion of the brain is activated to produce aura sensations. On the basis of firing patterns of limbic neurons recorded by microelectrode techniques in patients with temporal lobe epilepsy, only 14% of neurons at the epileptogenic zone are estimated to increase their firing rate in an aura. The corresponding estimate for a subclinical seizure is 7% and for a clinical complex partial seizure 36% (43). In agreement, an ictal–interictal subtraction single photon emission computed tomography study of isolated auras showed no clear dominant or reproducible areas of hyperperfusion in most cases (44).
Although an aura reflects activation of functional cortex by a circumscribed seizure discharge, the seizure discharge frequently spreads. A seizure that spreads along a single functional area as the postcentral gyrus produces the sensory equivalent of a jacksonian march. An aura can also spread across different functional regions. A seizure that starts in the primary visual area of the occipital lobe and spreads to the temporal limbic structures may present with initial transient blindness followed by other sensations referable to the temporal lobe.
Multiple sensations can occur even when seizure activity is relatively confined to one region, as at the start of temporal lobe seizures. Anxiety, epigastric, and “indescribable” sensations commonly precede the more complex phenomena of déjà vu and other illusions of vision or sound (45). When multiple auras can be dissected out along a sequence, they likely correlate with propagation of the ictal discharge within the temporal lobe, as recorded in a subdural EEG study (46). In other cases, a time series cannot be discerned, and the multiple sensations seem to occur simultaneously or vary in order from one seizure to another in the same patient. An alternative explanation for multiple auras could be that they are secondary to activation of a distributed network of several functional areas (47). The temporal limbic system, with extensive connections to the septum, hypothalamus, temporal neocortex, insula, and parieto-occipital association cortex, is precisely such a system. In support of this hypothesis, electrical stimulation of temporal limbic structures by depth electrodes can produce different sets of sensations at different times, despite stimulation of the same contacts (11).
SOMATOSENSORY AURAS
Tingling, numbness, and an electrical feeling are common, whereas absence of sensation or a sensation of movement is less. A sensation that starts focally in an extremity or shows a sensory march, such as an ascent from the hand up the arm to the face, points to a seizure discharge in the primary somatosensory area of the contralateral postcentral gyrus (26). A clinically identical seizure may have started more posteriorly in “silent” parietal cortex and caused symptoms only after it spread to the postcentral gyrus. A primary somatosensory aura can be interrupted by clonic jerking of the affected part and reflects spread from the postcentral to the precentral gyrus. A seizure starting in the primary motor area of the precentral gyrus can also cause a somatosensory aura, rapidly accompanied by clonic motor phenomena. Indeed, the precentral gyrus accounted for 25% of the sites producing a somatic sensation during intraoperative stimulation of the rolandic cortex (26).
Somatic sensations with a wide segmental (whole limb or side of body) or bilateral distribution indicate seizure activity outside the primary somatosensory area. Seizures arising from or involving the second sensory area, situated in the superior bank of the sylvian fissure and parietal operculum (26,48), evoke somatic sensations of the contralateral or ipsilateral sides of the body or both. The sensation can be rudimentary, but second sensory auras include pain, coldness, and a desire for movement (26).
Seizures arising from the supplementary motor area were preceded by an aura in nearly half the patients in one study (49). Penfield and Jasper (26) elicited somatic sensations from the supplementary sensory area, a part of the mesial cortex in the interhemispheric fissure, posterior to the supplementary motor area. Extraoperative stimulation using chronically implanted subdural electrodes not only confirmed the existence of supplementary sensory areas but also showed that they intermingle and overlap with the supplementary motor area, so that the two regions can best be regarded as a single functional entity (50,51). Auras from the supplementary motor and sensory areas include nonspecific tingling, desire for or sensation of movement, and feelings of stiffness, pulling, pulsation, and heaviness. These sensations usually involve extensive areas of a contralateral extremity or side of the body or bilateral body parts. They may be perceived as a generalized body sensation as well. Penfield and Jasper (26) also elicited epigastric sensations on stimulation of the supplementary motor area.
Chronic recordings and stimulation studies of depth electrodes implanted into the posterior insular cortex revealed a fourth brain region that can give rise to contralateral somatosensory sensations (52,53). The sensations include those of tingling, electrical shock, heat, and sometimes pain. They can involve more localized or more extensive regions on the contralateral side of the body. Somatosensory auras occur in a small percentage of patients with temporal lobe epilepsy (17) (Table 10.2). Since the temporal lobe is devoid of representation for somatic sensation, these auras are thought to occur secondarily to propagation of the ictal discharge into the second sensory area, the insula, or postcentral gyrus.
As an aura, a general body sensation, including diffuse warm and cold thermal sensations, has little value in cortical localization, having been reported as seizure aura from all regions of the brain. Besides the supplementary motor area, the mesial temporal structures (54) have responded to stimulation with such diffuse sensations.
Ictal pain as aura can be classified according to the affected parts: cephalic, abdominal, and somatosensory. Ictal headache will be discussed with other cephalic auras and abdominal pain with epigastric aura. Painful body sensations may represent the initial aura or occur as a component of an aura or seizure. The pain may be sharp, burning, electric, cold, or cramp-like and may be focally to diffusely distributed. Pain as an isolated symptom is much less common than as an association of paresthesias and other somatic sensations (55,56). Some patients experience cramp-like pain with tonic muscle spasm of an affected part. Well-localized and unilateral ictal pain generally occurs contralateral to an epileptic focus in the postcentral gyrus or neighboring parietal lobe (55–59). Electrical stimulation of the postcentral gyrus can elicit contralateral pain (57,60). Resection of the parietal cortex with the epileptic focus has successfully abolished painful seizures (56,57). Other areas reported to produce painful somesthetic auras are the second sensory area (26,61) and insular cortex (53). The localization of heat, cold, warmth, and flushing is variable or poorly understood. When these sensations are focal and unilateral, the same cortical regions described above are presumed responsible. When they are felt over wide segmental areas, on both sides of the body or in a generalized distribution, they lack reliable localizing or lateralizing value.
There are further somatic sensory alterations, which are distinctive with possible localizing value. Pharyngeal dysesthesias of tingling and burning or laryngeal discomfort to the point of a choking sensation are uncommon auras, sometimes reported in patients with seizures arising from the insula (53) or spreading from the temporal lobe to the insula. Aura sensations of body deformation, when a limb or part of the body is felt altered in size, twisted, or absent, suggest ictal alteration of sensory integration, a parietal lobe phenomenon (33).
VISUAL AURAS
Spots, stars, blobs, bars, or circles of light, monochromatic or variously colored, implicate seizure activity in the visual areas of the occipital lobes and occur in a majority of seizure arising there (26,34,38). These stationary or moving images may be lateralized to the visual field contralateral to the involved lobe but also may appear directly ahead. When they are lateralized and move across the field of vision, the patient’s head may turn to follow them. Some patients describe darkness proceeding to blindness, which can also occur as a postictal phenomenon in those with visual auras. Such ictal blindness or amaurosis occurs in up to 30% of occipital seizures. An occipital seizure may propagate to the temporal lobe or the parietal cortex. In the former instance, a visual aura may be followed by psychic experiences, epigastric aura, or emotional feelings, whereas a somatosensory aura may follow in the latter case. Auras with formed visual hallucinations are discussed under psychic auras, as are visual illusions such as macropsia and micropsia.
AUDITORY AURAS
The auditory cortex lies in the transverse gyrus of Heschl in the superior bank of the superior temporal gyrus or temporal operculum. Electrical stimulation there and in the adjacent superior temporal gyrus produces simple sounds variously described as ringing, booming, buzzing, chirping, or machinelike (26). Similar elementary auditory phenomena are described with auditory auras. At other times, partial deafness may occur, with impairment of auditory comprehension if the language dominant hemisphere is involved. Auras with such characteristics suggest seizure activity in the superior temporal neocortex, Heschl gyrus, or temporal operculum (26,62) and are usually noted in patients with lateral neocortical temporal lobe epilepsy rather than mesial temporal lobe epilepsy. Auditory aura is a defining feature in patients with autosomal dominant partial epilepsy with auditory features associated with LGI1 gene mutation (63). If the sounds in an aura are lateralized to one ear, the focus is usually in the contralateral hemisphere. Because seizures can spread to other portions of the temporal lobe, auditory auras are frequently accompanied by other temporal lobe phenomena. Other auditory illusions and formed hallucinations are discussed later on in this chapter.
VERTIGINOUS AURAS
Stimulation of the posterior extent of the superior temporal gyrus can elicit feelings of displacement or movement, including rotatory sensations (26). True vertiginous auras are probably uncommon but may be localized to the posterior part of the superior temporal neocortex (62) and experienced as one of several aura sensations that may arise together or in succession. True vertigo must be distinguished from nonspecific dizziness, which, on questioning, may resolve to cephalic sensations, light-headedness, or sense of impending loss of awareness, none of which has reliable localizing value. Early reports of patients with so-called vertiginous seizures probably included a large number with such nonspecific dizziness (64).
OLFACTORY AURAS
Jackson and Beevor (65) reported a “case of tumor of the right temporosphenoidal lobe bearing on the localization of the sense of smell and on the interpretation of a particular variety of epilepsy.” The patient experienced a “very horrible smell which she could not describe.” The term “uncinate fits” has been used to describe seizures with this aura because pathologic lesions are frequently found in the medial temporal lobe. The smell of an olfactory aura is often unpleasant or disagreeable (26,66) with odors akin to burning rubber, sulfur, or organic solvents. However, the smell can be neutral or even pleasant (67). The incidence of olfactory aura is low at about 1% (see Table 10.1). Whether patients with this symptom are disproportionately likely to have temporal lobe tumor is open to debate (66,68), as nonneoplastic lesions such as mesial temporal sclerosis can also be found responsible (67,69).
Other than the medial temporal lobe, the olfactory bulb is the only structure that can produce an olfactory sensation on electrical stimulation, and rarely, seizures starting in the orbitofrontal cortex can produce an olfactory aura (27). Olfactory aura rarely occurs in isolation; gustatory or other sensations referable to the temporal lobe frequently occur together or follow.
GUSTATORY AURAS
Usually disagreeable, the taste experienced may be described as sharp, bitter, acid, or sickly sweet. The incidence is low (see Table 10.1). Penfield and Jasper (26) ascribed the representation of taste deep in the sylvian fissure adjacent to and above the insular cortex. Hausser-Hauw and Bancaud (70) localized gustatory hallucinations to the parietal or rolandic operculum. They also recorded spontaneous and electrically induced seizures from the temporal limbic structures that were associated with gustatory phenomena but believed that the aura resulted from seizure propagation to the opercular region. Temporal lobectomies failed to abolish the gustatory hallucinations in three of their patients. The course of seizures with gustatory aura depends on the site of the epileptogenic zone. Suprasylvian seizures are likely to involve salivation, second sensory area sensations, and clonic facial contractions. Seizures of temporal origin may have epigastric aura in addition and develop automatisms.
EPIGASTRIC OR ABDOMINAL AURAS
Under this heading are various sensations localized to the abdomen or lower chest that may move to the throat and head but rarely descend in the opposite direction. “Visceral” and “viscerosensory” are other terms to describe this aura. Commonly characterized as a feeling of nausea, epigastric aura may also be reported as butterflies in the stomach, emptiness, queasiness as “going over a hill,” tightness, and churning; occasionally, it may be painful (58,71). This aura is frequently associated with or preceded or followed by other sensory, psychic, emotional, or autonomic phenomena (72). The sensation cannot be considered secondary to altered gastroesophageal function, as direct intraesophageal and intragastric pressure recording showed its occurrence with and without peristalsis (72,73). Although an abdominal aura is most common in temporal lobe epilepsy, it has been associated with epilepsies from all lobes of the brain (see Tables 10.2 and 10.3). Epigastric sensations can be elicited in epileptic and nonepileptic individuals by electrical stimulation of the amygdala, hippocampus, anteromedial temporal region, sylvian fissure, insula, supplementary motor area, pallidum, and centrum medianum of the thalamus (14,72).
CEPHALIC AURAS AND ICTAL HEADACHES
Cephalic aura includes a range of sensations felt in the head that includes dizziness, electrical shock, tingling, fullness, pressure, or other ill-defined sensations. It should not be confused with a somatosensory aura arising from the different cortical sensory areas. Moreover, electrical stimulation studies have provided no clear localization, and cephalic sensations have been reported as auras in focal seizures arising from all brain regions but may be more common in frontal lobe epilepsy (see Tables 10.2 and 10.3).
The relationship of headache to seizures is complex and is still the subject of considerable debate. Most common are postictal headaches, which can be diffuse or lateralized. Headaches can occur as an epileptic prodrome. Some patients with both migraine and epilepsy report that their seizures seem to be triggered by a headache. A headache of abrupt onset at the beginning of a seizure can be considered an aura or an ictal headache. An ictal headache can be pounding like a migraine but also sharp and steady. The pain may build gradually, but several patients studied with scalp or intracranial recording showed abrupt pain onset and offset synchronous with EEG seizure activity (74,75). Ictal headache is not well localized to any specific region and has been described also in generalized epilepsy. A lateralized headache is likely to be ipsilateral to the side of the epileptogenic focus (56,71,74,76). Many well-studied patients had temporal lobe epilepsy, possibly reflecting the increased likelihood of intensive presurgical EEG monitoring in this group. Patients with occipital lobe epilepsy represent the other major population with ictal headache, and when associated with a visual aura need to be differentiated from classic migraine. Ictal or postictal headache is often a striking symptom in benign epilepsy of childhood with occipital paroxysms (77) and in occipital seizures of patients with Lafora disease (78) and other progressive myoclonus epilepsies. It is possible that ictal headaches are not auras in the sense of a perception linked to localized neuronal activation, but the result of “an alteration in intracranial circulation either preceding the attack or coincidental with its onset” (26), akin to what occurs during migraine aura.
EMOTIONAL AURAS
Ictal fear describes pronounced anxiety or intense terror that is out of proportion to, and separable from, the understandable apprehension that can accompany any seizure. In some patients, the fear resembles a real-life experience, such as suddenly finding a stranger standing close behind, or is associated with an unpleasant psychic hallucination of past events. Others seemingly localize the sensation to the chest or stomach in association with an epigastric aura (79). Ictal fear may be accompanied by symptoms and signs of autonomic activation, such as mydriasis, tachycardia, and hyperventilation, and behavioral manifestations of screaming, calling for help, and agitated movements. An aura of fear has been linked to temporal lobe epilepsy (80) and can be elicited on stimulation of the mesial temporal structures (28,81). An aura of fear can be difficult to separate from a panic attack when it occurs in isolation, and correct identification relies on the associated ictal clinical and EEG findings.
Elation and pleasure are infrequent auras. The preictal happiness and ecstasy reported by Dostoyevski are cited as an example. Electrical stimulation of the left amygdala appeared able to produce either pleasant or unpleasant emotions, while right amygdala stimulation induced mainly negative emotions of fear and sadness (82). But in general, pleasurable sensations are rarely elicited by electrical stimulation. Depression as an aura or ictal phenomenon is debatable. In the largest series, reported by Williams (79), many of the patients had depression that lasted for hours to days, making it likely that this state constituted a prodromal mood change rather than an aura.
PSYCHIC AURAS
In 1880, Hughlings Jackson (2) described “certain psychical states during the onset of epileptic seizures” that included “intellectual aurae…reminiscence…dreamy feelings…dreams mixing up with present thoughts…double consciousness…‘as if I went back to all that occurred in my childhood’… These are all voluminous mental states and yet of different kinds.” Both Gowers (1) and Penfield and Jasper (26) included emotional auras under the heading of psychic auras. Others used the term “experiential” phenomena for these events (28,81).
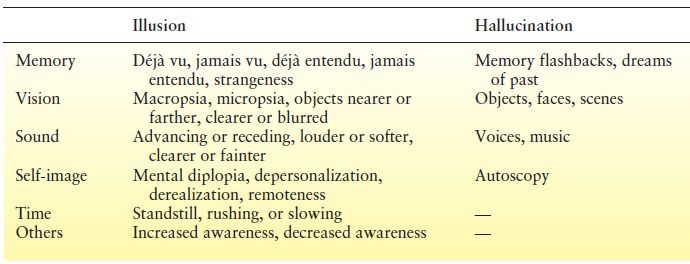
An illusion results from faulty interpretation of present experience in relation to the environment. Aware of the error in perception, the patient has “mental diplopia” in the jacksonian sense. A hallucination is a lifelike experience unrelated to present reality. Psychic hallucinations include dreamlike events or memory flashbacks that are complex and “formed,” in contrast to the elementary “unformed” hallucinations that characterize excitation of the primary sensory areas. Nevertheless, epileptic patients invariably sense that the hallucinations are not real.
The nature of psychic auras is as varied as their complexity. Many attempts at classification have been made (Table 10.4), but it may be fruitless to adhere to an overly rigid categorization of these rich phenomena that offer glimpses into the workings of human consciousness. For example, déjà vu can be considered an illusion of familiar memory; the converse, when what should have been a familiar visual experience becomes unfamiliar, is called jamais vu. The corresponding auditory illusions are déjà entendu and jamais entendu. Despite reports that psychic auras can occur with focal seizures from elsewhere in the brain, the consensus ascribes them to epileptic activation of the temporal lobe. Penfield and Perot (83) found that the sites eliciting psychic phenomena during acute intraoperative stimulation were nearly all in the lateral temporal neocortex, particularly along its superior border, and only occasionally in basal or mesial temporal regions. In contrast, later studies (28,84,85) identified both mesial temporal limbic (amygdala, hippocampus, entorhinal cortex) and temporal neocortex structures as capable of producing psychic phenomena, even in the absence of an electrical afterdischarge. To reconcile these differences, Gloor (47) proposed a hypothesis based on the model of a neuronal network with reciprocal connections—in this case, between the limbic structures and the temporal neocortex. Psychic phenomena arising “from the activation of matrices in distributed neuronal networks” could presumably be elicited from different locations within the temporal lobe, including temporal isocortex and various limbic structures. Signal analysis during déjà vu upon stimulation of the entorhinal cortex in epileptic patients found increased EEG signal correlations between medial temporal structures (86).
Table 10.4 Psychic Auras
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








