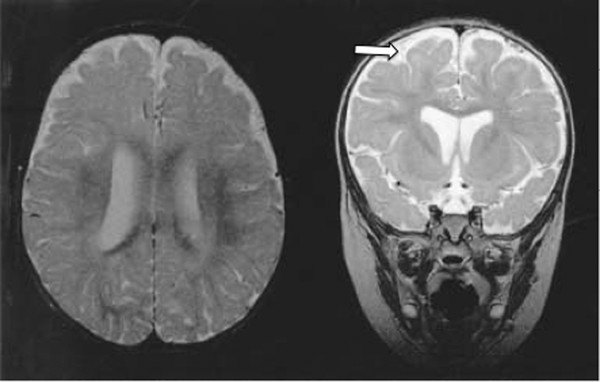
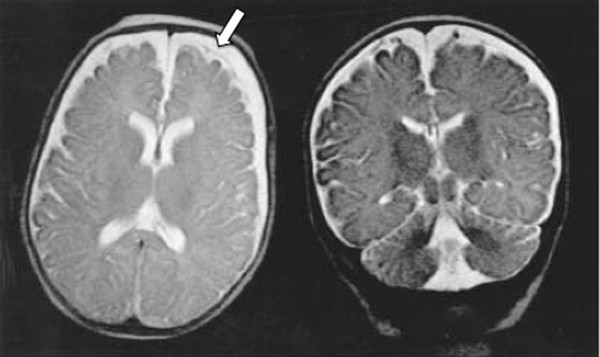
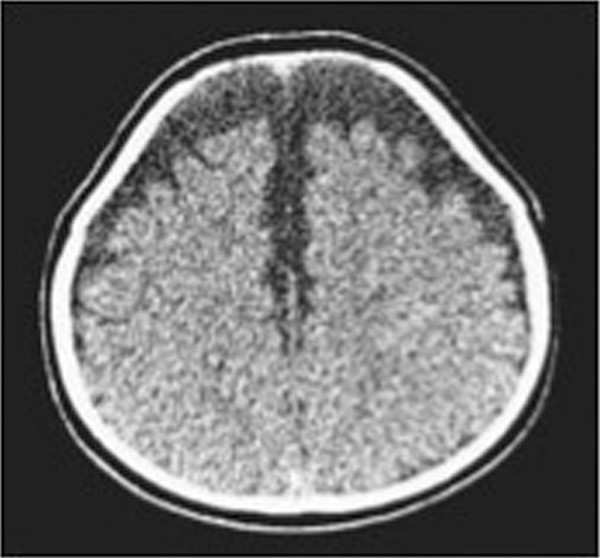
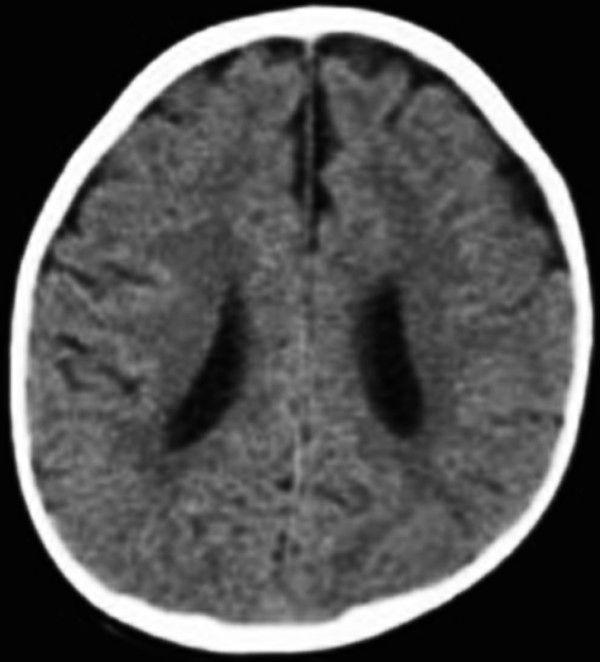
Extracerebral Fluid Collections in Infants Many terms, such as benign subdural fluid collections of infancy, benign subdural hygroma, subdural effusion, benign external hydrocephalus (BEH), abnormally enlarged subarachnoid space, and subarachnomegaly, are in use. Many more terms, such as benign subdural collections of infancy, benign extra-axial fluid of infancy, and benign enlargement of the subarachnoid space, attempt to designate the condition as benign based upon radiologic studies alone. The terms benign external hydrocephalus, idiopathic external hydrocephalus, extraventricular obstructive hydrocephalus, and pseudohydrocephalus megalocephaly include the word hydrocephalus despite minimal or no ventricular enlargement and minimal or no rise in intracranial pressure (ICP). The evolution of progressively better imaging modalities for the extracerebral spaces likely spurred on the process to refine and rename the visualized anatomical spaces and fluid collections over time, resulting in the multitude of terms used for the same or similar conditions. The nomenclature also likely reflects different theories of etiology and outcomes.1 The terms are summarized in the box “▶ Current and Historical Terms Used to Describe Enlargement of the Subarachnoid Spaces.” Current and Historical Terms Used to Describe Enlargement of the Subarachnoid Spaces Benign external hydrocephalus External hydrocephalus Benign extra-axial fluid collections of infancy Idiopathic external hydrocephalus Extracerebral fluid collections of infancy Pericerebral cerebrospinal fluid collections Primitive megalencephaly Benign obstructive hydrocephalus Subarachnomegaly Subdural effusion Subdural hematoma Subdural hygroma Subdural fluid collections of infancy Subdural fluid collections Pseudohydrocephalus megalocephaly Extracerebral intracranial fluid collections of childhood Benign enlargement of subarachnoid spaces Extracerebral fluid collections in infants reside in the spaces between the pia of the brain and the arachnoid (the subarachnoid space) and in the potential space between the arachnoid and the dura (the subdural space). Imaging of these collections in the infant is usually obtained for the evaluation of a large or enlarging head, as well as many other presentations. Idiopathic enlargement of the subarachnoid space is commonly considered benign with no clinical or radiographic features suggestive of high ICP. The term benign external hydrocephalus (BEH) is the one most commonly used in the published literature. It is used in this chapter to refer to the constellation of findings presented above, although many other names have been applied to this entity. It is important to note that the word hydrocephalus is somewhat of a misnomer because BEH is not generally considered a pathologic or surgical diagnosis and is usually self-limited. The anatomy of the extracerebral space can be conceptualized in layers. The pial surface of the brain marks the inner limit of the extracerebral space. The next layer is the arachnoid. Between the pia and the arachnoid is the subarachnoid space, which interdigitates into the cerebral sulci and overlies the cerebral gyri. The subdural space is the potential space outside the arachnoid and underneath the dural lining of the calvarial space. The subarachnoid space is incompletely divided by arachnoid trabeculae.2 The arachnoid and the arachnoid trabeculae are draped over pial vascular anatomical structures to maintain a relatively intact space overlying the entire supratentorial and infratentorial pial surfaces. The arachnoid and arachnoid trabeculae then expand into the extra-axial spaces to create cisterns. The surface cerebrovascular tree of both large and medium-size arteries and veins is contained within the subarachnoid space. The arterial half of the subarachnoid vasculature enters the subarachnoid space essentially upon entering the cranial vault and subdural space. The arterial vasculature comes from both the carotid circulation and the vertebral circulation. The arterial tree is spread over the pial surface and penetrates the brain parenchyma at intervals to supply blood to the intraparenchymal capillary circulation of the brain. Cerebral venous flow then goes in the other direction from the intraparenchymal capillary bed out of the brain parenchyma into the folds of the sulci and then into the subarachnoid space. Here, the veins coalesce and insert into large intradural venous sinuses. The venous subarachnoid vascular tree pierces through the arachnoid surface in many places into the true subdural space to feed directly into dural veins and venous lakes. These can be found as the veins enlarge and move closer to the large venous sinus structures, such as the superior sagittal sinus and the petrosal sinuses along the temporal bone.3 The Virchow-Robin spaces of cerebrospinal fluid (CSF), which are around the small blood vessels in the intraparenchymal space, may in fact be an extension of the subarachnoid CSF spaces along the brain-penetrating portions of the arteries.4 The relationship of these spaces to the enlargement of the subarachnoid space is not well understood. The subdural space is a potential space in which the only recognized structure between the arachnoid and the dura is a segment of dural bridging veins. There may be a small amount of CSF from an incompetent arachnoid membrane or from tracking along the traversing veins. The total length of the extra-arachnoid segment tends to increase as these veins approach the large venous sinuses. Any significant collection of fluid or other material, such as blood, in the subdural space is pathologic. There is no universally accepted or generally known function for the subdural space or the material within it. Magnetic resonance (MR) imaging is the gold standard imaging modality for identifying the subarachnoid space versus the subdural extra-arachnoid space.5–8 Particularly on T2-weighted sections, traversing cortical veins can be visualized within subarachnoid fluid collections at the cerebral convexities; this is referred to as the “cortical vein” sign5,9 (▶ Fig. 14.1). Fig. 14.1 Enlarged subarachnoid spaces. Axial and coronal T2-weighted high-resolution magnetic resonance images of the brain demonstrating enlargement of the subarachnoid space. Note the visible arachnoid layer, with most of the vessels (arrow) beneath it and only the dura above it. The arachnoid membrane overlying the subarachnoid space cannot be seen on the MR image unless pathologic fluid or other material exists on top of it in the subdural space, as in chronic subdural hematoma (▶ Fig. 14.2). Fluid in the subarachnoid space can clearly be differentiated from fluid or other material in the subdural space by the presence of a visible arachnoid membrane. Fluid in these spaces can differentiated from CSF based on MR imaging signal intensity.8 The ability of MR imaging to discern various densities of protein within the fluid allows a much more detailed determination of the exact anatomical location of CSF versus other types of fluid.5 Fig. 14.2 Chronic subdural hematoma. Axial and coronal T2-weighted high-resolution magnetic resonance images of the brain demonstrating bilateral chronic subdural hematomas in the late phase. Note the visible arachnoid layer (arrow) displaced downward by a fluid layer between it and the dura. Cortical vessels are not visualized within the fluid of this chronic subdural hematoma. Any significant amount of fluid in this space is pathologic. The subarachnoid space takes the shape of the gyri of the underlying brain, whereas the inferior boundary of the subdural space is smooth. Because of arachnoid trabeculae at the cortical sulci, the subdural space cannot interdigitate into the sulci. In cases of chronic subdural hematoma, the cortical vein sign is not present. Computed tomography (CT) as a modality for identifying extracerebral fluids is less sensitive than MR imaging because the vessels of the subarachnoid space are more difficult to visualize. CT can identify 90% of subdural fluid collections, whereas MR imaging is estimated to be 100% sensitive.10 In infants with open fontanels, the distance between the dura and the pial surface of the brain can easily be assessed by transfontanel ultrasonography.11,12 However, the examination is limited to the cone of ultrasonographic vision through the fontanel, as well as being limited by artifact from the skull bone. Clinically, it is difficult to draw conclusions about the exact anatomical location of the fluid in the extracerebral space or the nature of that fluid without MR imaging or, when needed, analysis of a fluid sample drawn percutaneously. Intravenous contrast neuroimaging studies show the rapid influx of contrast medium from CSF into the subarachnoid fluid spaces but not into fluid in the subdural extra-arachnoid compartment.13 The frontal subarachnoid spaces are enlarged beyond the upper defined limits for age in the setting of benign extracerebral fluid collections, and the cortical vein sign is visible on T2-weighted MR imaging. The ventricles are generally normal or moderately enlarged in size. The interhemispheric fissure is often but not always wider than normal; as well, the third ventricle and basal cisterns are larger than normal.14–17 Benign extracerebral fluid collections can be distinguished from global cerebral atrophy because the latter is associated with widening of the cerebral sulci globally and, not concentrated in the nondependent subarachnoid space, such as the frontal areas visualized in a supine patient.15 As well, cerebral atrophy is not associated with increasing head circumference. There is no consensus on the definition of normal subarachnoid space size. There is no significant difference between reported subarachnoid space sizes in male and female children.18–20 In neonates, the maximal upper measurement limit of the subarachnoid space width is reported to range from 3.3 to 5 mm.18,20,21 In infants younger than 1 year old, the maximal upper limit of normal subarachnoid space width is reported to range from 4 to 10 mm.16,19,22–24 Upper values for the interhemispheric fissure width range from 6 to 8.5 mm, and for the distance between the lateral wall of the superior sagittal sinus and the cortical surface (sinocortical width), they range from 2 to 10 mm.1,22–26 Variations in the size of the subarachnoid spaces are observed during growth, with the upper limit of normal width reported to increase from birth to 7 months,19 to decrease from 1 to 2 years of age,23 and to become essentially absent after 2 years of age.27–29 Fluid within the subarachnoid space in the nonpathologic state is CSF. Other fluids that can be present within the subarachnoid space endogenously are blood products or pus from a purulent infection of the surface of the brain. Fluid within the subdural space that is purely extra-arachnoid can be CSF transudate, blood (such as from rupture of a subdural bridging vein), blood transudate, or purulent material from an extra-arachnoid subdural abscess. Blood in its acute state in the subarachnoid or subdural space can be solid or gelatinous clot. The treatment of acute subdural hematoma or acute subarachnoid hemorrhage is outside the scope of this chapter and is discussed elsewhere in this volume. Once subdural or subarachnoid blood lyses to become a proteinaceous liquid, it falls within the rubric of an extracerebral fluid collection. Nongelatinous hemorrhage within the subarachnoid space tends to resolve over days to weeks because the subarachnoid space is a low-flow CSF space,30,31 whereas hemorrhage in the subdural space can take much longer to resolve because of the absence of CSF flow. Rupture of the arachnoid membrane, from trauma or surgical manipulation, fuses the subdural and the subarachnoid space for a time before the brain itself has the opportunity to scar to the underside of the dura, which may frequently happen. In that situation, in which the anatomical boundaries are blurred, the fluid mixture is more difficult to diagnose but can be some combination of CSF, blood, and pus. Blood in the subdural space undergoes a transition from solid clot to eventual resolution by lysis and resorption. During this progression, the blood can become bounded with subdural membranes, which are a fibrous encapsulation of the original clot that is vascularized.32,33 There is extensive literature on subdural membranes and their development and resolution. The membranes tend to enhance with gadolinium.34,35 The membranes also elaborate tissue plasminogen activator (tPA) and bradykinin, which can cause further or recurrent hemorrhage, spilling more and more blood into the subdural space.36,37 This creates a laminar hierarchy of hemorrhages in various states of resorption, with intervening membranes that become progressively thinner with time. The anatomical localization of these membranes also becomes farther and farther away from the dura, which appears to be the origin of their blood supply.38 The stages of resolution of subdural hematomas have been well described in the literature.39,40 Infection within the subdural space can be gelatinous, fluid, or a combination of both. The most accurate term for this infection is subdural empyema. Subdural empyema can involve both the subdural space and the subarachnoid space, which is observed on imaging and during intraoperative inspection.41,42 It is frequently associated with sinusitis and is thought to migrate along the valveless veins in the subdural space.42,43 The term subdural effusion has been used to describe a hypodense fluid accumulation associated with meningitis, such as in the case of Haemophilus influenzae infection.44 The term subdural effusion has also been applied to hypodense subdural fluid collections in the setting of trauma, adding ambiguity to the terminology used for these lesions. No matter what scheme of nomenclature is used, the anatomical components of the extracerebral space are as presented, and the fluid can comprise only CSF, blood (either acute or during a phase of resolution), or an exudate from a blood clot or an abscess. This concept allows a simplified interpretation of the nomenclature and of the pathologic entities involved. A number of theories about the pathophysiology of BEH exist, although the etiology is not completely understood. The most common theory is that the arachnoid villi are not mature, with a resultant inability to absorb CSF effectively, which leads to CSF accumulation in the subarachnoid space.45 With a relative impediment to the low-pressure resorption of CSF from the subarachnoid space through the arachnoid into the superior sagittal sinus, the subarachnoid space enlarges. The ventricles of the infant with an enlarged subarachnoid space may also appear to be mildly dilated and at the upper limit of normal.46,47 The sylvian fissures and sulci are also slightly enlarged. As the cranial sutures are not fused in this age group and the skull is compliant, a marked increase in ICP is generally avoided, whereas macrocephaly is common. Heredity is suggested to play a role, as more than 40% of children with BEH are reported to have a familial form, with one or more close relatives being macrocephalic and having a head circumference at or near the 98th percentile5,28,46,48–52 BEH also reported to occur in twins and triplets. The phenotype may be related to delayed maturation of the arachnoid villi.15 Follow-up evaluation of many of these patients shows that the parenchymal brain volume appears to conform to a normal configuration by 3 or 4 years of age,28,29 supporting spontaneous resolution of an underlying problem, such as immature arachnoid villi, which mature at age 18 months.1,53 Supporting the idea of mildly impaired CSF resorption is the observation that conditions with increased central venous pressure, such as right-sided heart failure and pulmonary disease, enlarge the subarachnoid spaces, presumably through impeded resorption.15,54,55 (▶ Fig. 14.3) Additional correlation between increased CSF spaces and rigid venous outflow impedance comes from observations in craniosynostoses, plagiocephaly, and hydrocephalic achondroplastic dwarfs.56–59 Fig. 14.3 Axial noninfused computed tomographic scan of the head of a 3-month-old girl with right-sided heart failure and a bulging fontanel demonstrating enlargement of the subarachnoid space. Enlarged subarachnoid spaces may be on the spectrum preceding internal hydrocephalus in children with communicating hydrocephalus if the arachnoid granulations remain dysfunctional, with CSF accumulating in the subarachnoid space and then subsequently in the ventricular system over time.29,60 However, there is a distinction between delayed maturation of the arachnoid villi with regression of the problem after the age of 18 months and agenesis of the arachnoid villi with necessity for surgical CSF diversion.53,61 Another theory for the pathophysiology of BEH is craniocephalic disproportion, with the skull transiently growing at a faster rate than the brain. The brain growth is believed to be slow in these children, and after a lag period, the brain begins again to fill the cranium.15,50,62,63 The developmental pathophysiology of benign enlargement of the subarachnoid spaces is not completely understood. In our experience, BEH has not progressed to frank hydrocephalus with an enlarged fluid space under inappropriately high pressure unless it is complicated by infection or hemorrhage; however, such rare occurrences have been reported in the literature.46,64–66 In the setting of presumed mild malabsorption of fluid, a small amount of blood mixed into the subarachnoid CSF can cause the pressure to increase, so that extracranial shunting is eventually required, as in absorptive hydrocephalus with ventricular enlargement having other causes.29 Purely subdural bleeding might not lead to such a problem because it would not mix with the CSF in the subarachnoid space. Blood found in the subdural space is generally thought to come from the disruption of bridging veins, which penetrate the arachnoid after draining the cortical surface and then insert into the underside of the dura or directly into a dural venous sinus structure.67 Various issues around the acute management of subdural hematoma are discussed in other chapters. As an extracerebral fluid collection, subdural blood can be of small volume or can mix with the CSF of a ruptured arachnoid membrane. As a purely subdural collection, blood will coagulate and then organize into a mature clot. At that point, it may become encapsulated by membranes and then be slowly reabsorbed. A chronic subdural hematoma is not stable because ongoing bleeding from the membranes can increase the size of the fluid collection and turn a relatively small initial hemorrhage into a lesion causing mass effect.68 It can also increase the tension on already stretched veins, leading to repeated rupture from minor trauma or inertial changes. As the blood is resorbed, the fluid collection will become less dense, a change visible on CT and MR imaging; however, it can easily be differentiated from BEH by the lack of visible subarachnoid vessels (i.e., no cortical vein sign). The vessels in the subarachnoid space would not enter into an isodense chronic subdural hematoma in its late phase. A connection between BEH in childhood and the development of normal-pressure hydrocephalus (NPH) in the elderly has been suggested, with a two-hit hypothesis of childhood BEH followed by deep white matter ischemia leading to symptoms in later years.69 Patients with NPH have significantly larger intracranial volumes than control subjects on imaging,70 and 20% of patients with NPH have head circumference measurements over the 90th percentile.71 The possible link between BEH and later NPH has yet to be fully explored and may offer future insights into natural history and management. BEH has been associated with developmental delay50,72–76 and an increased theoretical and observed risk for subdural hematoma.7,77–79 Other reported associations include prematurity and intraventricular hemorrhage,80–83 meningitis,81,84 metabolic disorders,85,86 and certain medications, such as chemotherapeutic agents.87 BEH has also been associated with raised venous pressure, such as in systemic disease, thoracic conditions, and right-sided heart failure.15,54,55 Rigid venous outflow obstruction and thus raised venous pressure in craniosynostoses, plagiocephaly, and achondroplasia have been reported to cause external hydrocephalus.56–59 Syndromes associated with enlarged subarachnoid spaces include achondroplasia, Sotos syndrome, Beckwith syndrome, Weaver syndrome, and Goldenhar syndrome.28 Fetal MR imaging studies show that 19% of those with mild ventriculomegaly and large subarachnoid spaces prenatally later carried a diagnosis of BEH as infants.47 The prominent subarachnoid spaces seen prenatally are distributed more posteriorly; this pattern reflects the formation of the subarachnoid space as a cavitation of the primitive meninges from the ventral to the dorsal portion of the neural tube.88 Although isolated subdural hematoma without a known intervening trauma can be a sign of nonaccidental trauma, it appears that our knowledge of the pathophysiologic mechanisms for subdural hematoma in the setting of preexisting BEH is incomplete. There are ongoing investigations on the infant shaking impact syndrome.89–91 BEH may predispose toward subdural hematoma from an impact that would otherwise not cause a subdural hematoma in a child with normal-size subarachnoid spaces. The proposed etiology is stretching of bridging veins in the enlarged subarachnoid spaces. The risk for developing subdural hematoma is reported to be higher in children with BEH after minimal or no trauma to the head.6,7,78,79 Because of these observations, subdural hematoma is not considered pathognomonic for nonaccidental trauma in situations in which BEH is present. A careful search for other evidence in the context of a full evaluation for nonaccidental trauma must be made in the patient with BEH and subdural hematoma before child abuse is diagnosed. In the absence of other evidence, the diagnosis of nonaccidental trauma cannot be based solely on the presence of a chronic subdural fluid collection in a child with BEH and no history of significant head impact. The incidence and prevalence of BEH are not known. A review of the records of a tertiary pediatric neurology practice notes that 0.6% of patients were diagnosed with BEH.92 Approximately two-thirds of children with BEH are male.48,93 BEH is reported to be the most common cause of macrocephaly in infants in developed countries.28,94 Most studies report a familial pattern, and from 40 to 80% of children with BEH have at least one close relative with macrocephaly and a head circumference measuring above the 98th percentile.5,28,46,48–52 BEH has been reported in twins and triplets, suggesting a genetic component.31,95 The inheritance pattern is postulated to be autosomal-dominant or multifactorial, with a gene exerting an effect during a limited period of susceptibility in development, such as a delay in the maturation of arachnoid villi.15,60,96 Extracerebral fluid collections can present like any intracranial mass lesion. In BEH, the presentation is recognized by a large head circumference that may be crossing percentile markings in the first several months of life.28,62,76 The fontanel will generally be soft and will be concave upon sitting. The sutures will not be split. Occasionally, the child may also present with positional plagiocephaly, which has a correlation with BEH58 (▶ Fig. 14.4). Fig. 14.4 Axial noninfused computed tomographic (CT) scan of the head of a 6-month-old boy with occipital plagiocephaly. CT scan demonstrates enlarged subarachnoid spaces, which are reported to be associated with positional plagiocephaly.
14.1 Introduction and Nomenclature
14.2 Anatomy of the Extracerebral Space
14.3 Imaging of the Subdural Extra-arachnoid Space
14.3.1 Radiographic Anatomy and Imaging Characteristics of Benign Extracerebral Fluid Collections
14.3.2 Normal Measurements of the Subarachnoid Space
14.4 Identification and Pathophysiology of Fluid within the Extracerebral Spaces of the Infant
14.4.1 Identification
14.4.2 Pathophysiology
14.4.3 Associated Conditions
14.4.4 Implications for the Evaluation and Diagnosis of Nonaccidental Head Trauma
14.5 Epidemiology
14.6 Clinical Presentation of Extracerebral Fluid Collections in the Infant
Extracerebral Fluid Collections in Infants
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








