Chapter 15 Extramucosal Diseases of the Head and Neck
SALIVARY GLANDS
Anatomy
A portion of the parotid gland extends deep to the plane of the facial nerve (identified radiographically by the stylomandibular tunnel from the styloid process to the ascending ramus of the mandible) and is termed the deep lobe of the parotid. The superficial lobe extends from just under the skin and usually has an accessory tongue of tissue that passes over the masseter muscle. These lobes do not actually exist; it is an arbitrary distinction dividing the gland into anatomic sections based on the facial nerve. The importance of differentiating the deep and superficial portions of the parotid stems from the different surgical approaches to tumors in each section and the relationship to the facial nerve (Fig. 15-1). If a lesion is in the superficial portion of the parotid gland, it is usually approached from an external periauricular incision, the facial nerve is dissected deep to the mass to ensure its safety, and the lesion is plucked (excised) from the gland superficial to the nerve. If a mass in the deep portion of the parotid is well defined and noninfiltrative, it is also approached from the same incision; the facial nerve is dissected and then lifted up to shell out the deep lobe mass. However, if the lesion is infiltrating through the deep portion and may encase the nerve, the approach may be combined with a parapharyngeal space approach via a neck incision below the ear. Unfortunately, with infiltrating deep lobe lesions the facial nerve must often be surgically sacrificed (if it has not already been sacrificed by the tumor itself).
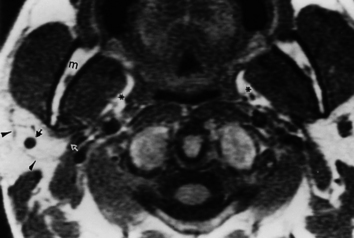
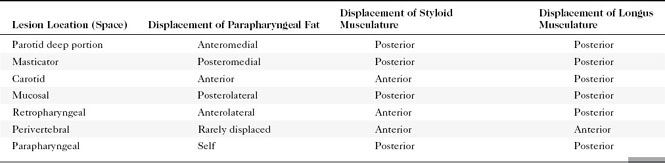
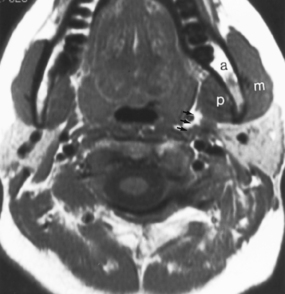
Deep parotid space lesions displace the parapharyngeal fat anteromedially and maintain a fat plane between the lesion and the mucosal surface of the pharynx. The parapharyngeal fat is a good marker for telling in what space a lesion is located (Table 15-1).
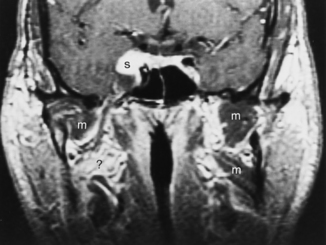
The submandibular space (Box 15-1) encompasses the tissue below the mucosa of the floor of the mouth yet above the fascia connecting the mandible to the hyoid bone. As such it contains the sublingual compartment with the mylohyoid muscle, sublingual gland, the deep portion of the submandibular gland, the associated ducts, and the corresponding neurovascular structures. Below the sublingual space is the submaxillary space with the main superficial portion of the submandibular gland, the level Ia and Ib lymph nodes.
The submandibular gland is located in and below the floor of the mouth, deep to the angle of the mandible (Fig. 15-2). The submandibular gland secretes seromucinous saliva, as opposed to the parotid gland, which secretes serous saliva. In addition, the pH of the saliva produced by the submandibular gland is more alkaline and the fluid is more viscous. The duct of the submandibular gland is called Wharton’s duct, and it drains on either side of the frenulum of the floor of the mouth. The duct has a tighter orifice but is wider than Stensen’s duct and is more easily traumatized in the mouth. The duct of the submandibular gland courses anteriorly and superiorly before reaching its orifice.
Congenital Disorders
Branchial Cleft Cyst
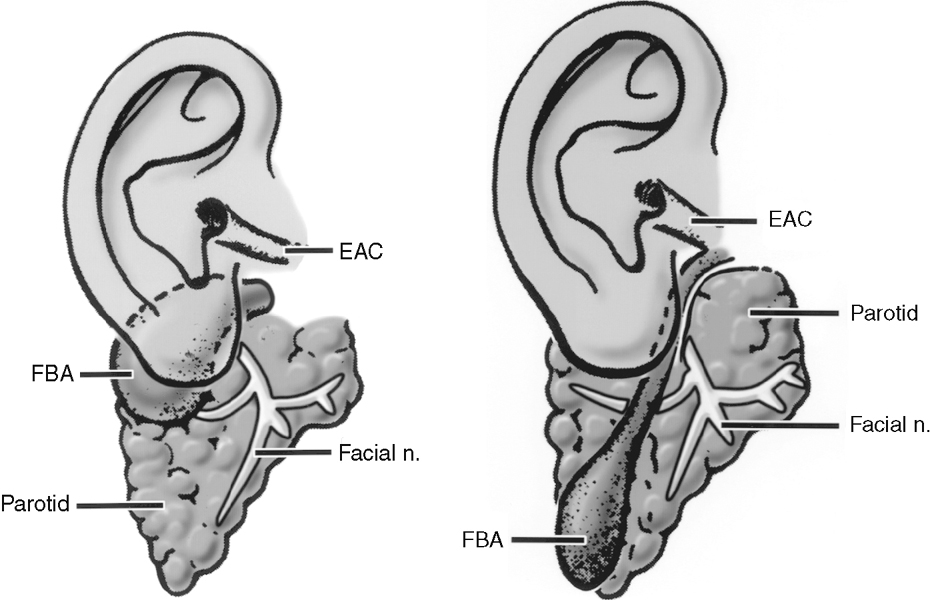
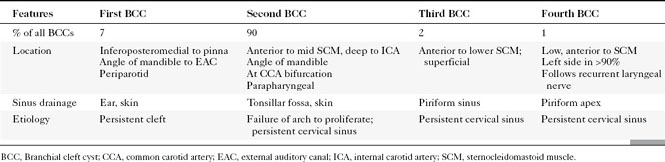
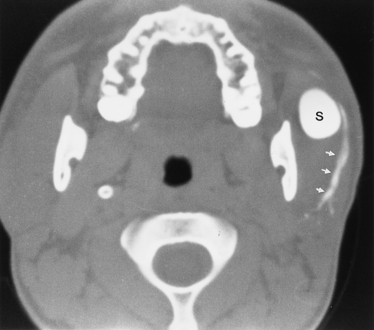
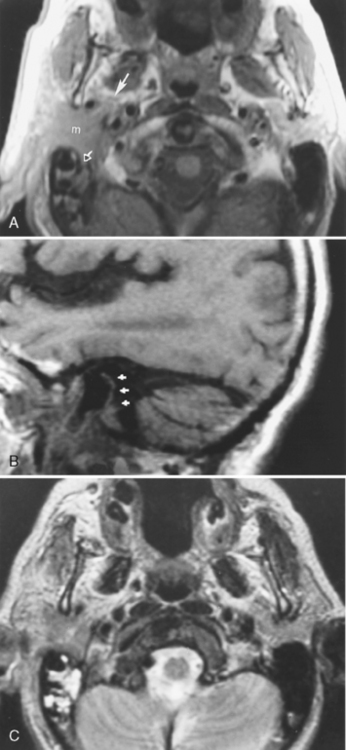
First branchial cleft cysts (BCCs) classically occur in the parotid gland or around the external auditory canal (Box 15-2). Several classifications of first BCCs have been developed, including Arnot type I (intraparotid cyst) and Arnot type II (cyst in the anterior neck that may drain with a tract through the deep portion of the parotid gland to get to the external auditory canal) (Figs. 15-3 and 15-4). They may drain into the external auditory canal and are of the same density and intensity as cerebrospinal fluid (CSF) unless otherwise infected or traumatized. Because second BCCs are so much more common, they probably outnumber first BCCs in the periparotid location (Table 15-2). Adjacent inflammation may be present, and the whole complex may simulate an infiltrative process. Fistulization to the bone-cartilage junction of the external ear may occur with first branchial cleft anomalies.
Inflammatory Lesions
Calculous Disease
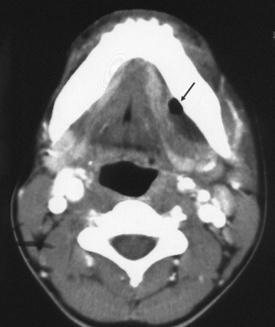
Calculous disease is the most common benign condition to affect the salivary glands. Because the submandibular gland secretes a more mucinous, viscous, alkaline saliva and the wider duct must drain in an uphill direction with greater possibility of stasis, calculi occur most commonly in the submandibular gland and duct (Fig. 15-5). In fact, submandibular gland calculi outnumber those in the parotid gland by a “calculation” of four to one. Sublingual gland calculi and minor salivary gland calculi are extremely uncommon. Although most of the calculi associated with the salivary glands are radiopaque, a small percentage (20%) may not be so radiodense as to be visible on plain films.
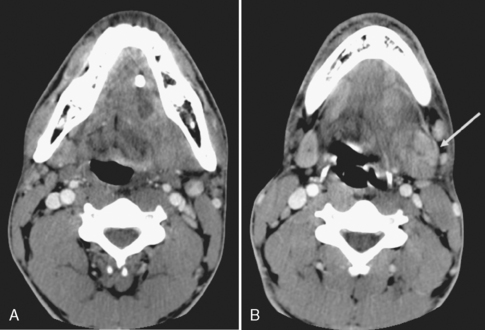
Patients who have calculous disease usually have painful glands, exacerbated by chewing foods that precipitate salivation. If the clinician suspects calculous disease, the usual workup includes plain films to evaluate for large radiopaque calculi. If no calculi are identified by plain films, or if the patient has a fever associated with a painful salivary gland and an abscess is suspected, unenhanced CT should be done because it is more sensitive for the detection of calcification and inflammatory masses (Fig. 15-6). Remember that magnetic resonance (MR) imaging is less sensitive to calcified or noncalcified tiny calculi; larger calculi can be seen as low-intensity areas on T2-weighted scans, but flow voids may simulate calculi. In the instance of a calculus that is not radiopaque on CT, one might be forced to perform conventional sialography (cannulation of the salivary duct with injection of contrast). MR sialography is a new technique that neuroradiologists have stolen from the MR cholangiopancreatography protocol of the body MR guys. One can use either a single-shot fast spin-echo heavily T2-weighted sequence that highlights the ducts alone or perform a high-resolution 3D fast spin-echo T2-weighted sequence that can be prescribed through the gland and duct. Sialography is useful in demonstrating strictures of the ducts after passage of a calculus and intraluminal filling defects from nonopaque stones. Ductal strictures may predispose to recurrent calculous disease. However, the use of sialography for diagnosing calculi has dramatically decreased because CT is so effective here.
With a classic clinical history suggesting stones, treatment may proceed without antecedent imaging.
Sialadenitis
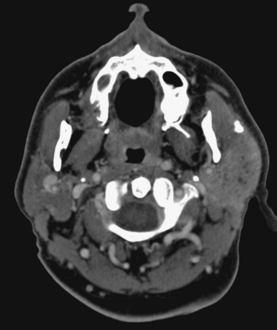
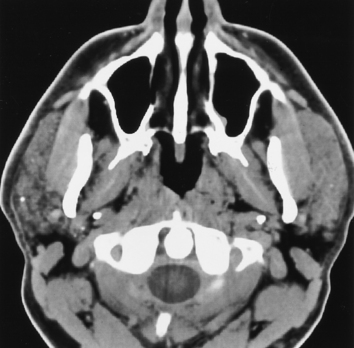
Sialadenitis refers to glandular inflammation, whereas sialectasis refers to dilatation of ductal spaces. Sialadenitis is often associated with sialectasis, or dilatation of the ductal system. The most common cause of these conditions is calculous disease. Microabscesses within the parotid tissue may be seen in a person who has sialectasis or sialadenitis. They are identified on CT as areas of low density with peripheral rim enhancement and on MR as areas of very bright signal intensity on T2WI of the salivary glands. Microabscesses often are multiple and may be a source of painful parotid glands with fever. Abscesses may develop around the mandible, sublingual gland, or submandibular gland in association with dental infections (Fig. 15-7).
Sialodochitis
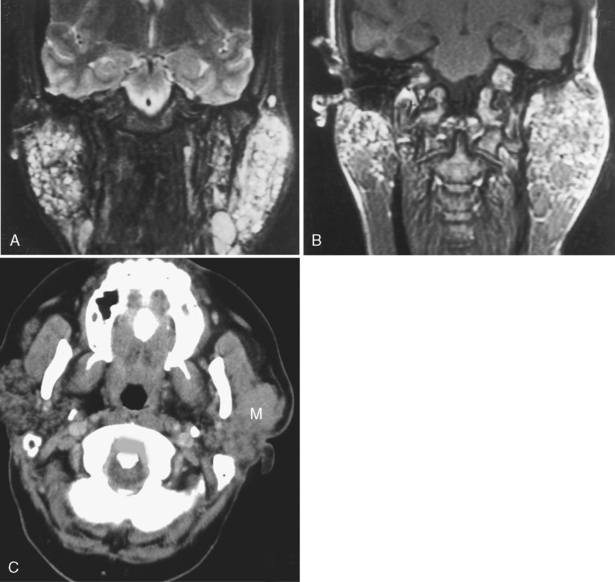
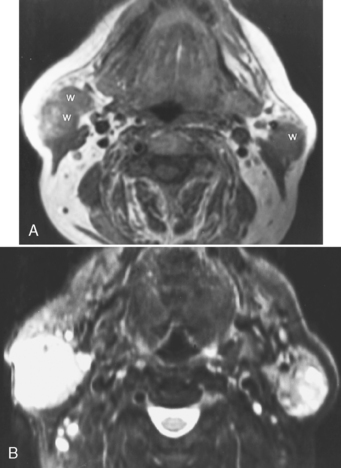
Sialodochitis refers to inflammation of the main salivary ductal system. A number of autoimmune conditions may cause sialadenitis and sialodochitis. When the process is limited to the salivary glands without other associated findings, the disease is termed Mikulicz disease or, in the most recent classification, Sjögren type 1 disease. This is an autoimmune disorder that causes chronic sialadenitis and sialodochitis and leads to fibrous salivary gland tissue (primarily of the minor glands) with resultant dry mouth. This disorder usually affects middle-aged women. When the disease is associated with a collagen vascular disease (most commonly rheumatoid arthritis more so than systemic lupus erythematosus) and involvement of the lacrimal glands, the disorder is classified as Sjögren syndrome (Sjögren type 2). Sjögren syndrome is an autoimmune disorder that causes dry eyes, dry mouth, and arthritis. Patients with Sjögren syndrome have tenfold increased risk of lymphoma, which may have its first manifestations in the parotid glands. The lymphoma is usually of the non-Hodgkin variety and may affect any other area of the head and neck as well (Fig. 15-8). Often these patients are scanned to survey for the possibility of lymphoma.
The cross-sectional imaging appearance of parotid glands in patients who have Sjögren disease may range from normal to a dried-up, scarred-down, atrophic gland, to one with lots of large or tiny cysts and nodules within it, to one with a dominant mass within it. This looks very much like HIV-related parotid disease (Fig. 15-9).
Lymphoepithelial Lesions
Since the ascent of acquired immunodeficiency syndrome (AIDS) in the young population, lymphoepithelial lesions of the parotid gland have become much more common. These may include purely cystic lesions or solid lesions of lymphoid aggregates (Fig. 15-10). Therefore, in a younger patient with multiple lesions in the parotid gland you should consider lymphoepithelial lesions, as opposed to multiple Warthin’s tumors. The differential diagnosis also includes multiple intraparotid lymph nodes and lymphoma. The lymphoepithelial lesions of the parotid have been associated with HIV seropositivity, and the presence of these abnormalities may predate the infection that classifies the patient as having AIDS. Associated findings with HIV-related parotid disease include diffuse generalized lymphadenopathy in the neck and prominence of adenoidal and tonsillar tissue. Bone marrow signal intensity on T1-weighted imaging (T1WI) may be lower than normal. When the lymphoepithelial lesion is cystic, it has low density on CT and signal intensity characteristics of CSF on T1WI and T2WI. However, the lymphoepithelial solid nodules may have a more variable density and signal intensity on cross-sectional imaging.
Sialocele
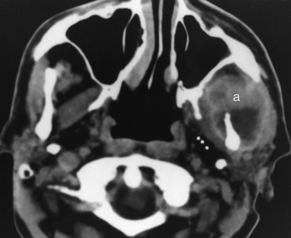
A sialocele refers to a collection of saliva that communicates with the parent duct in a manner similar to that of a pharyngocele or a laryngocele filled with fluid (Fig. 15-11). The most common cause of sialoceles is penetrating trauma, although blunt trauma may also cause disruption of the duct and leakage of salivary contents into the parenchyma and outside the gland. This most commonly occurs in the parotid gland, either from a punch to the side of the face or from a stab wound. The entity is distinguished from a pseudocyst because it communicates with the parent duct and is not lined by fibrous tissue.
Ranula
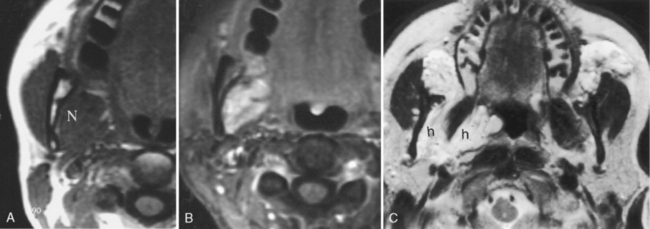
Another entity, more fully described in Chapter 14 in the discussion of oral cavity lesions, is the ranula. This is a postinflammatory cystic lesion that results from obstruction of either the sublingual or submandibular duct and that produces a cystic mass either confined by the mylohyoid muscle (simple ranula, epithelial lined) or extending to the submandibular region (a plunging ranula, not epithelial lined) (see Fig. 14-22). A ranula has also been termed a “mucus escape cyst,” a mucus retention cyst, and a mucocele of the sublingual gland or neighboring minor salivary glandular tissue. The simple ranula is usually addressed transorally but may be treated with resection or, in some cases, marsupialization. The lingual and hypoglossal nerves must be carefully identified during the operation. A plunging ranula may be excised through a transcervical submandibular incision with a neck dissection. This allows complete resection of the cyst and will help spare the lingual and hypoglossal nerve. Alternatively, the surgeon may excise the sublingual gland transorally and pack the cyst or place a drain in it. By treating the gland, some believe the plunging cyst will resolve on its own.
Benign Neoplasms
Pleomorphic Adenoma
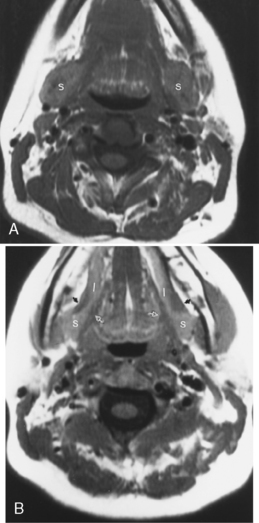
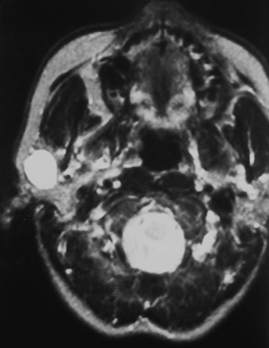
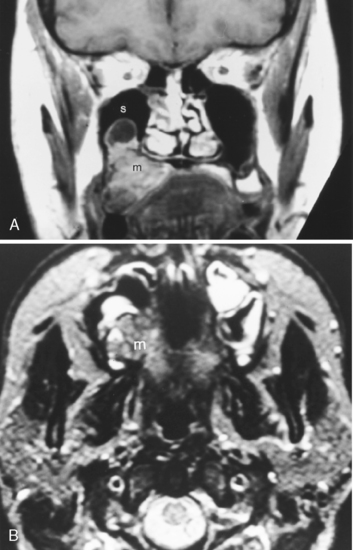
Nearly 80% of benign parotid neoplasms are pleomorphic adenomas (Box 15-3). Pleomorphic adenomas, also known as benign mixed tumors, occur most commonly in middle aged women. Most pleomorphic adenomas are well-defined lesions that commonly appear solid and round. Pleomorphic adenomas are well identified on both CT and MR against the fatty background of the normal adult’s parotid gland (Fig. 15-12). On CT, the lesions have density similar to that of muscle and demonstrate mild to moderate enhancement. With a delay, one may see an increase in the degree and homogeneity of enhancement in parotid pleomorphic adenomas. On MR, the lesions are best identified on T1WI amid the bright signal of the parotid fat; however, they are usually seen on T2WI as very bright lesions (add that to your 80% rule—80% of bright lesions in the parotid are pleomorphic adenomas). Additional MR findings include a complete capsule (often low intensity on T2WI) and a lobulated contour. Pleomorphic adenomas inconstantly have cystic degeneration or calcification within them. Because the incidence of calcification is so much lower in other types of parotid tumors, the presence of calcification nonetheless suggests pleomorphic adenoma.
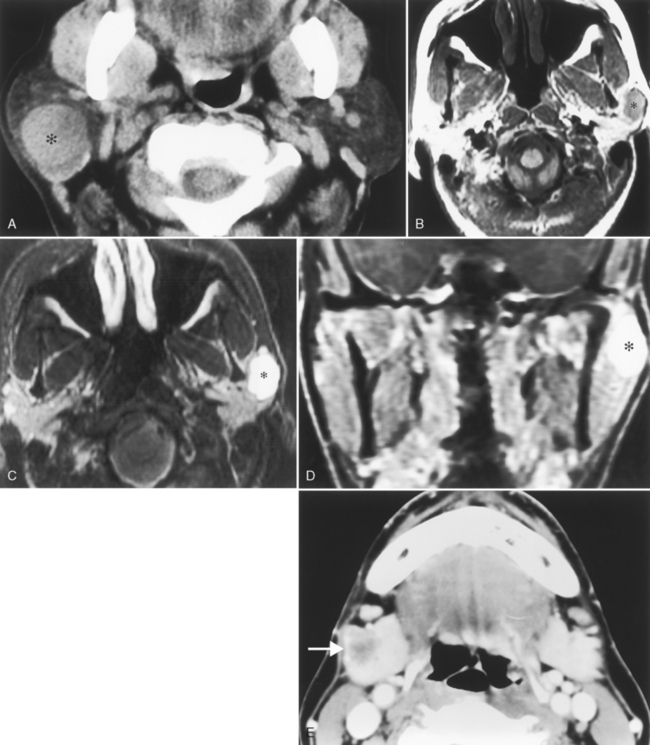
Figure 15-12 Pleomorphic adenoma. A, The well-defined mass (asterisk) in the superficial portion of the right parotid gland turned out to be a pleomorphic adenoma, following the 80% rules of the parotid gland (see Box 15-3). B, A different patient had a mass (asterisk) identified incidentally on this T1-weighted image (T1WI) in the left parotid gland. C, The brilliant bright signal on T2WI, in the face of a that does NOT l ook like a cyst, suggests a pleomorphic adenoma (asterisk)—80% of the time. D, Avid enhancement (asterisk) on postcontrast T1WI is characteristic as well. E, Monomorphic adenoma of the right submandibular gland (arrow), low in density, well defined, easily removed.
Warthin’s Tumor
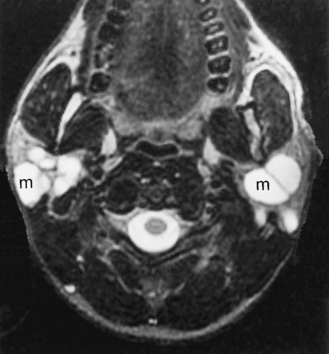
Warthin’s tumors are also known as cystadenoma lymphomatosum. These tumors are nearly exclusive to the parotid gland and are the most common multiple and bilateral tumors in the parotid (Box 15-4). As opposed to pleomorphic adenomas, which are generally seen in middle-aged women, Warthin’s tumors are most commonly seen in elderly men. Warthin’s tumors may have a tumoral cyst and favor the parotid’s tail. These lesions are entirely benign and show no evidence of malignant transformation. Therefore if a fine-needle aspiration identifies a lesion as Warthin’s tumor, surgeons may conservatively watch the tumor rather than remove it. On MR, the lesions are well seen on T1WI opposite the high signal intensity of the parotid gland (Fig. 15-13). However, the signal intensity on T2WI is often heterogeneous and variable and may overlap that of the bright signal of pleomorphic adenomas or the darker intensity of malignancies of the parotid gland. Warthin’s tumors, like oncocytomas, have increased uptake on technetium-99m pertechnetate nuclear medicine scans. Therefore, if fine-needle aspiration is equivocal or nondiagnostic, recommend a technetium nuclear medicine scan to make the diagnosis of Warthin’s tumors.
Malignant Neoplasms
Patients with parotid malignancies usually have a palpable, discrete, painless mass (98% of cases). Other presentations include facial nerve dysfunction (24%) and cervical adenopathy (6%). Facial nerve paralysis associated with a parotid mass usually means a malignancy is present. Of the malignancies to cause a facial nerve paralysis, adenoid cystic carcinoma and undifferentiated carcinoma predominate, with an incidence of 17% to 26%. The mean delay in reporting the mass to a physician is 3 months. The T staging of malignant salivary gland lesions is outlined in Box 15-5. Nodal staging and metastasis staging (M0 none, M1 present) follows that of the aerodigestive system (see Box 14-12).
Box 15-5 Classification of Salivary Gland Malignancies
* Note: Extraparenchymal extension is clinical or macroscopic evidence of invasion of soft tissues. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes.
From Greene FL, Balch CM, Fleming ID, et al (eds): AJCC cancer staging manual, ed 6, New York, 2002, Springer-Verlag, p 70. Used with permission.
Mucoepidermoid Carcinoma
The most common malignant lesion of the parotid gland is mucoepidermoid carcinoma; in the submandibular, sublingual, and minor salivary glands it is adenoid cystic carcinoma. Mucoepidermoid carcinomas, like squamous cell carcinoma, can be graded from low to high, and the prognosis varies with the grade. Mucoepidermoid carcinomas account for 30% of all salivary gland malignancies, and 60% of them occur in the parotid gland (Fig. 15-14). Mucoepidermoid carcinoma is the most common pediatric salivary gland malignancy. Thirty-five percent of salivary gland neoplasms in children are malignant; of these, 60% are mucoepidermoid carcinomas.
Adenoid Cystic Carcinoma
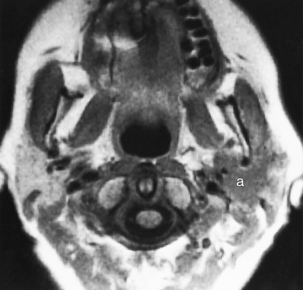
Adenoid cystic carcinoma, the second most common primary malignancy of the parotid gland and the most common tumor of the submandibular, sublingual, and minor salivary glands, is notorious for its propensity for perineural spread (50% to 60%) and persistence despite “complete surgical removal.” Similar to the mucoepidermoid carcinoma, variable intensity occurs with the T2WI, which allows a weak guess at histology (Fig. 15-15). Noncystic masses in the parotid gland have muscular CT density, low intensity on T1WI, and mild to moderate enhancement. An adenoid cystic carcinoma of the parotid gland may spread via the ramifications of cranial nerve VII retrograde into the temporal bone or may spread via the auriculotemporal branches of cranial nerve V to the Meckel’s cave region through the foramen ovale. One would think that spread via cranial nerve IX because of the innervation could occur, but that has not been seen by the authors … yet. Again, adenoid cystic carcinomas may be very well defined within the parotid gland, and the diagnosis of a malignancy may not be suspected before biopsy. In the other salivary glands adenoid cystic carcinoma generally demonstrates perineural extension along the branches of the second and third divisions of cranial nerve V. This cancer is a relentless, slow-growing tumor whose prognosis is generally measured in terms of decades rather than 5-year survival rate because of its prolonged course.
Squamous Cell Carcinoma
Squamous cell carcinoma may be seen within the parotid gland. Sometimes it is difficult to tell whether the squamous cell carcinoma is present secondary to invasion of lymph nodes from a primary site outside the parotid or is intrinsic to the parotid gland (Fig. 15-16). How it gets there is mysterious; it is presumably caused by metaplasia of the ductal columnar epithelium into squamous cells. This same difficulty lies with lymphoma of the parotid—is it a primary parotid tumor or secondary spread? When multifocal in the parotid, it is generally accepted that the squamous cell carcinoma is probably within lymph nodes in the parotid gland. A search for a primary tumor should be undertaken. The overlying skin and ear are the primary sites that drain to the parotid gland; however, the parotid lymph nodes may be involved with diffuse lesions such as lymphoma. Squamous cell carcinoma does not generally occur in submandibular, sublingual, or minor salivary glands as a primary site, although it certainly spreads from adjacent mucosal surfaces or lymph nodes. As mentioned in Chapter 14, obstruction of submandibular or sublingual gland ducts may be a presenting symptom of floor of mouth cancers.
Squamous cell carcinomas are virtually always hypointense on T2WI unless necrosis coexists.
Adenocarcinoma
Adenocarcinomas may also arise within the parotid gland, sublingual gland, submandibular gland, or minor salivary glands. This lesion generally has a worse prognosis than that of mucoepidermoid carcinoma and adenoid cystic carcinoma (Box 15-6). This tumor is derived from the glandular tissue itself as opposed to ductal tissue. Signal intensity is variable, depending on mucinous, cystic, or solid contributions. Some adenocarcinomas occur from malignant degeneration of pleomorphic adenomas.
MASTICATOR SPACE
Anatomy
The masticator space is defined by layers of the deep cervical fascia and encompasses the muscles of mastication (the medial and lateral pterygoid, masseter, and temporalis muscles) as well as the condyle and ascending ramus of the mandible, branches of the external carotid artery and third division of cranial nerve V, and venous branches from the jugular system (Fig. 15-17). Of the muscles of mastication, the small lateral pterygoid has a primary function of opening the mouth, whereas the bulky medial pterygoid, masseter, and temporalis muscles serve to keep the mouth closed.
How do you identify a lesion as being within the masticator space? When a masticator space lesion is present, the parapharyngeal fat is displaced posteromedially and may be infiltrated along its anterolateral aspect (see Table 15-1). Even if the lesion does not arise within the muscles, bone, or cranial nerve V, you will be able to identify a masticator space lesion by this characteristic displacement of the parapharyngeal fat.
Congenital Disorders
Lymphangiomas (loculated, infiltrative, multicystic, nonenhancing lesions with high signal on T1WI and T2WI) and venous vascular malformations that enhance, are solid, and are dark on T1WI and bright on T2WI may also infiltrate the masticator space (Fig. 15-18). Both of these lesions are usually evident at birth.
Diseases that may infiltrate the muscles of mastication are listed in Box 15-7.
Inflammatory Lesions
Odontogenic Lesions
Most of the inflammatory lesions of the masticator space relate to infections of odontogenic origin. Therefore, abscesses around the teeth, osteomyelitis of the mandible, and cellulitis associated with carious teeth are the prime offenders in this category (Fig. 15-19). Occasionally, cellulitis and myositis of the masticator space develop as a result of penetrating injuries, superficial facial infections, or adjacent parotitis. Most inflammatory lesions of the masticator space can be readily identified on CT or MR. On CT, look for thickening of the adjacent muscle, infiltration of the nearby fat, subcutaneous tissue, or skin with a strand pattern to it. If an abscess has developed, it will appear in a fashion similar to abscesses elsewhere, with a low-density center and a peripheral enhancing rim. On MR, inflammatory lesions, because of their high water content from edema, are very bright on T2WI. There are some exceptions to this rule, namely actinomycosis (because of sulfur granule deposition) and fungal infections (because of paramagnetic iron and manganese accumulation).
Bruxism and Atrophy
Bruxism may develop in patients who constantly gnash their teeth. This is a fancy word for (usually) bilateral enlargement of the muscles of mastication. It occurs most commonly idiopathically but may develop as a result of malocclusion, excessive chewing, or clenching the teeth. Rarely, this may be a unilateral phenomenon. Alternatively, one may see atrophy of the muscles of mastication when one has a cranial nerve V abnormality (Fig. 15-20). This may be caused by lesions in the peripheral branches of the third division of cranial nerve V or by loss of central input to the motor portion of the trigeminal nerve from lesions in the frontal cortex. Perioperative injury may cause denervation atrophy, as can trauma. With denervation atrophy, one may see enhancement after gadolinium administration or high signal intensity on T2WI in the muscles of mastication. Neurogenic tumors may also induce these imaging characteristics.
Fibrous Dysplasia
Most of the lesions of the mandible are odontogenic in origin. As explained in the discussion of the oral cavity (see Tables 14-1 and 14-2), radicular cysts from carious teeth and dentigerous cysts associated with unerupted teeth are the most common causes of cystic lesions of the mandible.
Temporomandibular Joint Syndrome
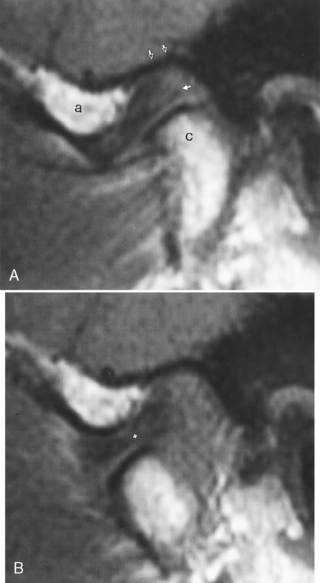
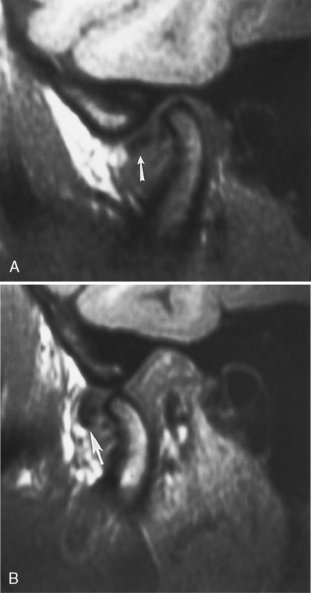
The temporomandibular joint (TMJ) falls under the rubric of the masticator space. The TMJ may be the source of chronic facial or head pain. TMJ (or chronic maxillofacial pain) syndrome is seen in women nine times more frequently than men, is often precipitated by a traumatic event, and is very poorly understood. Presently, most imaging is performed with MR where the meniscus, condyle, glenoid fossa, and surrounding soft tissues may be evaluated. Rarely, arthrography may be performed through injection of the joint under fluoroscopy. The meniscus has an anterior triangular band and a larger posterior band, which are joined in the middle by an intermediate zone (Fig. 15-21). The posterior band is attached to the posterior joint by the retrodiskal tissue, or bilaminar zone. The meniscus should be centered over the condylar head in open- and closed-mouth positions with the posterior margin of the posterior band between the 11 and 12 o’clock point of the condyle in the closed-mouth position. The joint itself has an anterosuperior compartment and an inferior compartment, which usually do not communicate.
Anterior meniscal dislocations are the most common type in patients with TMJ complaints. In this setting the meniscus’ posterior band is dislocated anteriorly from directly over the condyle. This displacement is more than 10 degrees anterior to the 12 o’clock position, more like the 9 or 10 o’clock position. It may be far in front of the condyle in the closed-mouth position on sagittal T1WI (Fig. 15-22). The dislocation may reduce on opening (often with a clicking sound—the “opening click”) and may redislocate on closing (a closing click). The timing of the opening click may correlate with the degree of anterior dislocation of the meniscus. Alternatively, the meniscus may remain anteriorly dislocated even on opening. The location of the disk in front of the condyle may restrict the joint’s motion (a closed-lock situation).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree