Extraoperative Functional Mapping
Prasanna Jayakar
Ronald P. Lesser
Introduction
Focal cortical resections are now frequently performed in patients with partial seizures or hemispheric tumors. During resection, it is important to spare “essential” cortical regions whose removal would lead to an unacceptable neurologic deficit. Essential areas generally can be spared by limiting the resections to certain anatomic landmarks. For example, a left anterior temporal lobectomy restricted to approximately 4 to 5 cm from the temporal tip will usually spare language cortex.10,22 There is considerable variability, however, in both the location and the extent of essential cortex in different subjects. The anatomic approach thus does not allow the maximal removal of abnormal tissue, nor does it provide complete security that eloquent cortex is not resected.24,44
Electrical stimulation has been used for greater than half a century to accurately map essential cortex intraoperatively. With development of the subdural electrode technology, extraoperative functional mapping became an option, especially in patients in whom invasive ictal recordings are necessary for planning resection. Responses to electrical stimulation have also been used by some epileptologists to confirm the location of epileptogenic tissue. This chapter reviews the role and technique of extraoperative mapping in patients undergoing cortical resections.
Effects of Electrical Stimulation
Electrical stimulation can produce a variety of effects. It may evoke “positive” responses such as localized movements from the primary or supplementary motor cortex, dysesthesia from the somatosensory cortex, phosphenes from visual cortex, or, rarely, “buzzing” from auditory cortex. Formed visual responses may be elicited from secondary visual cortex. Alternatively, stimulation may block function, presumably by sustained depolarization or activation of inhibitory systems. This effect cannot be appreciated in a quiet, resting patient and can only be demonstrated by having the patient engage in specific tasks during the stimulation. For example, stimulation of specific regions of the cortex can interrupt higher cortical functions such as those involved in receptive or expressive language. In areas such as the primary or supplementary motor cortex, both “positive” responses in the form of motor movements and “negative” responses such as arrest of movement or speech can be demonstrated.
In addition to clinical responses, stimulation also can provoke sustained electrographic afterdischarges, which generally last from seconds to minutes after cessation of the stimulus. Because afterdischarges are known to propagate to remote regions, functional localization is often felt to be more reliable when clinical responses are elicited without provoking afterdischarges. It is also generally assumed that the observed clinical response arises from cortex below the stimulated electrode or from the region between two closely spaced bipolar electrodes, an assumption that is supported by evidence from experimental models, although remote effects may occasionally occur.35,42
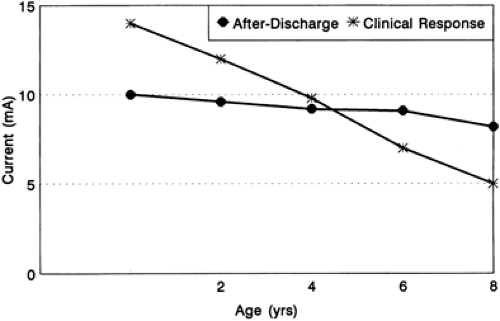
The thresholds for evoking both clinical responses and afterdischarges are usually higher in children than in adults.2,26,35 This difference is particularly striking in the first 2 years of life. Young children also differ from adults in that they rarely demonstrate clinical responses in the absence of an electrical afterdischarge.11,26 As illustrated in FIGURE 1, clinical response threshold usually exceeds the afterdischarge threshold in children younger than age 4 to 6 years, although this trend may occasionally be seen in older children and adults as well. This finding has important practical implications because the lack of a clinical response during an afterdischarge does not guarantee the absence of underlying eloquent cortex.26
Indications
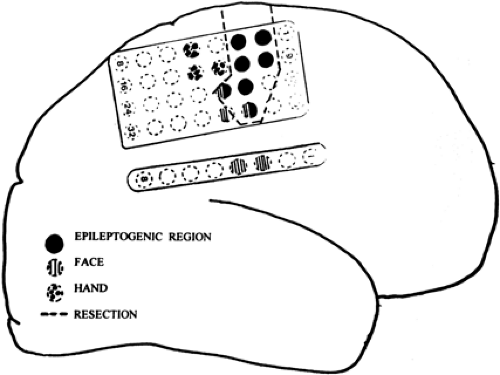
Functional mapping is used primarily to define essential cortex during surgeries for epilepsy or removal of structural lesions such as tumors or arteriovenous malformations (AVMs). Once eloquent cortex is precisely defined, the surgical resection can be accomplished to within 0.5 to 1.0 cm from it. In specific cases such as the one illustrated in FIGURE 2, one can assess the risk–benefit relationship and decide to resect a part of the motor cortex involved in the epileptogenic process. Functional mapping thus allows the removal of the most tumoral or epileptogenic tissue possible without incurring significant postoperative deficits.
Identification of Normal Cortical Function
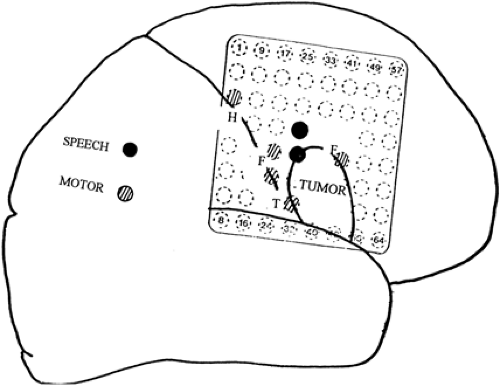
Mapping is most frequently indicated to define critical cortical regions subserving primary sensorimotor and language functions, which are often very localized in a patient but show considerable intersubject variability. This variability may be even more pronounced in patients in whom underlying lesions such as tumors cause considerable displacement of critical cortex. It is not unusual to find functional responses occurring over wide regions in a patchy distribution (Fig. 3), especially when a mass lesion is present. The thresholds for eliciting responses may also vary surrounding the tumor sites.
Language Cortex
Cortical stimulation of specific areas will block comprehension or other language-related activities such as spoken speech and writing abilities. Although language is most frequently represented in the left hemisphere, right hemispheric language dominance can occur even in patients who are motorically right
handed (Fig. 3). The majority of patients will demonstrate language cortex over both frontal and temporoparietal sites. However, in a few patients, essential language cortex is found only over the frontal or temporoparietal regions but not over both.46
handed (Fig. 3). The majority of patients will demonstrate language cortex over both frontal and temporoparietal sites. However, in a few patients, essential language cortex is found only over the frontal or temporoparietal regions but not over both.46
Localized regions of language cortex can be identified in children even as young as 2 1/2 years old (unpublished observation). Ojemann et al.46 reported both frontal and temporal language sites in children 4 to 10 years of age, including sites within 3 cm of the temporal tip. These findings indicate that a high degree of language localization occurs very early in childhood and suggest that mapping may have a role in planning resections even while the brain is still plastic.
Frontal Lobe.
In general, the frontal language area is anterior to but contiguous with the bulk of the motor and sensory area, although considerable overlap between these regions can occur.34,44 It is most frequently located at the posterior aspect of the inferior frontal gyrus or middle frontal gyri or both. However, language functions may occasionally be disrupted on stimulation of inferior frontal regions as far anterior as the pterion or even posterior to the rolandic sulcus. Writing capability is generally represented at sites overlapping those representing verbal speech, but in some patients they may be separate and involve the posterior part of the middle frontal gyrus.34,37
Temporal Lobe.
Stimulation of the posterior aspects of the superior or middle temporal gyrus in the dominant hemisphere can interrupt reading, naming, comprehension, and other language-related functions.36,44 Either single functions or groups of functions may be affected at single sites. Critical language cortex is usually located >4 cm from the temporal tip, although more-anterior locations are occasionally observed.
Stimulation in the adjacent temporo-parieto-occipital junction can affect nonverbal capabilities. For example, Morris et al.41 demonstrated all of the features of Gerstmann’s syndrome in this region, including right–left confusion and finger agnosia. Hart et al.21 reported an area where stimulation selectively interfered with the representation of size. These observations appear to support the idea that many types of information may be organized in a categoric fashion by the brain.
In addition to the temporo-parieto-occipital junction, language function may also be subserved by the temporal base.8,39 A variety of language functions can be altered at sites throughout the dominant temporal base, but unlike the convexity sites, resection of basal sites generally does not lead to significant permanent deficit.
Stimulation of some sites in the left lateral temporal cortex reportedly disrupts pathways involved in recent verbal memory. These sites cannot be consistently demonstrated in all patients, even after detailed testing extraoperatively, and the role of mapping memory cortex is yet to be fully defined.
Sensorimotor Cortex
Motor or sensory responses can be elicited by stimulating not only the primary sensorimotor cortex but also adjacent regions, which are not as critical and are termed the supplementary sensorimotor areas.
Primary Sensorimotor Cortex.
The primary motor and sensory homunculi do not necessarily conform to specific gyri on either side of the rolandic sulcus. Although the motor cortex is in general located anterior to sensory cortex, there is often an overlap, with some electrodes revealing both motor and sensory responses. Motor responses are usually elicited from a thin, strip-like region that varies in width between 1 and 2 cm. Wider motor representation of >3 cm also can be demonstrated, especially during extraoperative stimulation.53 Such wide representation may be attributable to atypical gyral orientation or technical factors such as current spread.
Motor responses in children show a developmental trend and become increasingly complex and well defined with age.2,11 Although hand and face regions can be defined, movements of an individual finger or the tongue are rare below age 2 years and become reliably elicited only after age 4 to 6 years. One could speculate that these findings may somehow reflect immaturity of cortical systems subserving fine finger movements or oroglossal coordination necessary for speech.
Stimulation of the lower rolandic region in children <1 to 2 years of age elicits bilateral rather than unilateral responses from the lower half of the face, suggesting that lower as well as upper facial muscles are likely to be innervated bilaterally in fetal and early postnatal life. Maturation would therefore lead to a loss of ipsilateral lower representation, probably through synaptic or axonal elimination. Such a hypothesis is consistent with the observation that the lower face is completely spared in prenatally acquired hemiplegia but is invariably involved when insults are acquired postnatally.32 On occasion, there can be ipsilateral as well as contralateral responses in adults as well, especially when stimulation is used to inhibit movement.
Supplementary Sensorimotor Area.
The supplementary sensorimotor area (SSMA) occupies mainly the superior frontal gyrus within the interhemispheric fissure, anterior to the primary motor cortex with variable extension onto the premotor convexity.38 Regions over the paracentral lobule, the precuneus, and even the adjacent cingulate gyrus, however, may reveal similar responses (Fig. 4). Stimulation of the SSMA can elicit unilateral or bilateral movements from both upper and lower extremities and the face. Stimulation at specific sites may interrupt ongoing motor activities, for example, those that produce alternating movements in the extremities or speech or writing. In general, the face and upper extremities are represented anteriorly and the feet toward the paracentral lobule.14,38
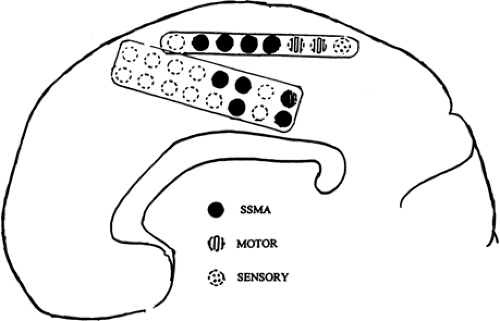 FIGURE 4. Functional map of the right interhemispheric cortex. The supplementary sensorimotor area (SSMA) is located anterior to but encroaches on primary motor and sensory regions. |
The SSMA is presumably involved in the planning and initiation of motor movements. Resection of this region on one side usually produces only transient alteration of movement or speech. From a practical standpoint, it is therefore important to differentiate the SSMA from the primary motor leg area located just posterior to it. Besides eliciting ipsilateral limb movements, SSMA response thresholds can be higher than those in the primary motor cortex. Time-locked responses to single-pulse stimuli reportedly occur only from the primary cortex and may help to further differentiate it from premotor areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree