tips and tricks
clinical features that raise suspicion for fshd
- Facial weakness and shoulder weakness with little or no lower extremity weakness
- Typical appearance of the shoulder: forward sloping shoulders, straight clavicles and axillary creases due to pectoral muscle atrophy
- Striking asymmetry in degree of muscle weakness
Figure 9.1. The left panel shows typical shoulder profile with straight clavicles, axillary creases due to pectoral muscle atrophy (a), and asymmetric winging (b). The right panel shows limited arm shoulder abduction to about 90° (c) with improvement to about 130° with manual fixation of the scapula (d).
(Reproduced from Tawil R, van der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve 2006;34:1–15, with permission from Wiley-Blackwell.)
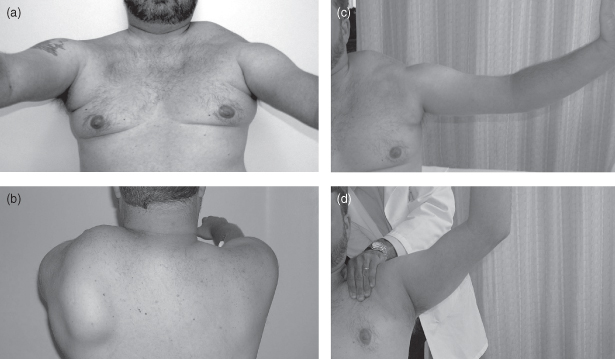
Although weakness is initially proximal in the upper limbs, weakness in the lower limbs most often begins in more distal muscles such as tibialis anterior, producing a foot drop. Core muscle weakness in the abdomen and back is an early, common, and often under-appreciated feature of FSHD. Weak abdominal muscles may result in a distended abdomen, out of proportion to body habitus, and in difficulty doing a sit-up. Paraspinal muscle weakness may also cause an exaggerated lumbar lordosis. On physical examination, selective involvement of the lower abdominal muscles may produce a striking upward displacement of the umbilicus with neck flexion in a supine position. This is known as Beevor’s sign and, in the setting of a suggestive history, is supportive of an FSHD diagnosis.
Weakness in FSHD tends to be asymmetric and the presence of striking asymmetry supports the FSHD diagnosis. Extraocular and bulbar muscles are typically spared. Respiratory failure due to neuromuscular weakness occurs in less than 5% of patients with FSHD, typically in wheelchair-bound patients with progressive kyphoscoliosis.
The most likely initial symptom is difficulty reaching above shoulder level, more pronounced on one side than the other. Foot drop is a less common presentation although invariably such patients have unnoticed shoulder weakness and scapular winging. Overall, deficits in FSHD tend to be chronic and slowly progressive, and consequently patients learn to adapt to significant weakness.
Extramuscular Manifestations
The two most common extramuscular manifestations in FSHD are: high-frequency hearing loss, occurring in 75% of affected individuals, and retinal vascular abnormalities, occurring in 60% of patients. In most patients neither the retinal nor the auditory involvement results in clinically significant impairment; however, recognition of their occurrence is important for physicians participating in the long-term care of these patients. Hearing loss, which is most frequently asymptomatic, may be severe in children with infantile-onset disease and thus require prompt intervention with hearing aids to avoid developmental delays in language. Also, in some of the most severely affected FSHD patients, the retinal vascular abnormalities may progress into an exudative retinopathy and resulting visual loss, a clinical sequence known as Coat’s syndrome. Screening of such patients with indirect ophthalmoscopy and treatment of significant vascular aneurysmal dilations can help arrest or prevent visual loss.
Prognosis
Prognosis tends to be inversely related to age of symptom onset. A child with significant periscapular weakness carries a worse prognosis than a 20 year old with only subtle facial weakness. In FSHD, the rate of progression is slow and typically remains steady. A subset of patients, however, reports a stepwise course where quiet intervals are followed by sentinel pain and then rapid deterioration in the involved muscles.
Individuals develop variable degrees of disability, with 20% of patients eventually becoming wheelchair bound. Patients with infantile-onset disease are frequently severely disabled, with the potential additional burden of hearing loss and visual loss compounding their limb weakness.
Life expectancy in FSHD is said to be normal because severe bulbar, respiratory, and cardiac involvement is rare. Nevertheless, unrecognized and untreated respiratory failure can lead to unexpected early mortality.
Genetics
Understanding the genetic mechanism of FSHD has been a challenge since 1992, when FSHD was first associated with a deletion of what appeared to be transcriptionally silent repetitive DNA elements in the subtelomeric region of chromosome 4q35. In over 95% of individuals with FSHD, there is a deletion of an integral number of macrosatellite repeats on 4q35. Normal individuals have 11–100 repeats, whereas patients with FSHD have 1–10 D4Z4 repeats. The absence of signs of FSHD in individuals with large deletions of 4q35 involving all D4Z4 repeats and the need to have at least one residual repeat in FSHD suggest a deleterious gain of function rather than a loss of function. Moreover, although D4Z4 contractions are necessary to cause FSHD, the contraction has to occur on a specific chromosomal background, known as A161, with specific DNA-sequence variations proximal and distal to the repeats. Moreover, contraction on this permissive background is also associated with a change in the chromatin state in the D4Z4 region from a nonpermissive to a permissive chromatin conformation. Recently, evidence for a unifying genetic model of FSHD was published.
 science revisited
science revisited
Two prevailing models for FSHD were pursued. One postulated that the D4Z4 contraction resulted in a change is expression of a gene from within the repeats. Indeed an open reading frame for a gene, DUX4, was present in each repeat, but it was difficult to demonstrate whether it was an expressed gene or a pseudogene. In the second model, contraction of the repeats influenced the expression of genes proximal to the deletion. Recent evidence supports the former model. The current model of FSHD pathogenesis is as follows: contraction of a critical number of D4Z4 repeats changes the chromatin structure to a euchromatic conformation which is more permissive of gene expression; the 4qA161 background contains a polyadenylation (poly(A)) sequence just distal to the last copy of the DUX4 gene. This poly(A) tail is essential in stabilizing mRNA for translation into protein. Thus, contraction of D4Z4 on a 4qA161 background triggers expression of a stable DUX4 mRNA and protein. The function of DUX4 remains uncertain, although early experiments indicate that it can interfere with muscle regeneration and make cells more susceptible to oxidative stress, leading to apoptosis.



