caution!
muscle biopsy
Muscle biopsy evidence of reversible statin myopathy can occur without any overt clinical symptoms and with normal creatine kinase (CK) levels. Therefore, the decision to proceed with a muscle biopsy must be carefully considered and alternative explanations for the patient’s symptoms should be explored. The treating physician should be fully aware of disorders that can predispose to cramping, leg heaviness/fatigue, and myalgia.
Lipid-Lowering Medications
With virtually all the lipid-lowering drugs (statins, fibrates, bile acid-binding resins, and ezetimibe) implicated in muscle toxicity, many have theorized that cholesterol is critical to the skeletal muscle cell membrane and that lowering cholesterol may explain the class effect. This rationale seems to account for the potential myotoxicity of lipid-lowering drugs at a superficial level. However, direct evidence is lacking. In fact, human skeletal muscle cholesterol content increased after 8 weeks of simvastatin therapy (80 mg/day) and did not change in response to atorvastatin (40 mg/day). The role of cholesterol depletion in lipid-lowering myopathy remains uncertain at this point and alternative mechanisms may account for the toxic effect of this class of drugs.
Statins
At the pharmacodynamic level (i.e. their site of action) all statins act similarly by selectively binding to the active site of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, inhibiting the enzyme competitively. However, at the pharmacokinetic level (i.e. absorption, distribution, metabolism, and excretion), the statins have metabolic differences related to their physiochemical properties, which in turn may translate into differences in myotoxic potential and the risks of interactions with other medications or toxins (Box 4.2). For example, medications that interact with the cytochrome P450 (CYP) CYP3A4 system may increase serum levels of some of the statins and must be used with caution. In addition to medications, there are number of comorbidities that predispose to statin myopathy.
Box 4.2. Drugs interacting with statins
Amiodarone
Azole antifungals
Calcium channel blockers (diltiazem and verapamil)
Ciprofloxacin
Colchicine
Cyclosporine
Hypolipemics (cholestyramine, gemfibrozil and other fibrates, niacin, ezetimibe)
HIV protease inhibitors
Macrolide antibiotics (clarithromycin, erythromycin, azithromycin)
Nefazodone
Histamine H2-receptor antagonists (cimetidine, ranitidine)
Sitagliptin (?)
Warfarin
 caution!
caution!
drug interactions with statins
The CYP3A4- and -2C9-dependent statins are subject to drug–drug interactions that could produce meaningful escalations in serum statin levels and cause myopathy. Therefore, ensuring normal liver and renal function and choosing non-interacting drugs will minimize the risk for myotoxicity. Indeed, a recent report, probing the adverse event reporting system of the Food and Drug Administration (FDA), demonstrated that the adverse event reporting rate ratio of simvastatin-induced rhabdomyolysis was 6.4 when comparing simvastatin-treated patients with and without a concomitant CYP3A4 inhibitor. Box 4.2 is a list of the various drugs known to interact with statins.
Adding another layer of complexity is the discovery that individual variation in the gene encoding human organic anion-transporting polypeptide C (SLCO1B1) may influence serum statin levels and be associated with statin myopathies, e.g. a single risk allele (Val174Ala) in SLCO1B1 accounts for 60% of the myopathic reactions to simvastatin.
The mechanisms responsible for statin-induced muscle breakdown are not known. Numerous risk factors have been identified. Much interest has focused on the role of coenzyme Q10 (CoQ10), also referred to as ubiquinone. CoQ10 is a fat-soluble compound that transfers electrons in the electron transport chain of the mitochondria. Statins lower CoQ10 levels in the blood and are thought to reflect an effect on low-density lipoprotein (LDL)-cholesterol. Statins, by blocking HMG-CoA-reductase, impair isoprenoid synthesis and this in turn should lower CoQ10 levels, but definitive evidence for this mechanism is lacking. Nevertheless, case reports have documented lactic acidosis in response to statin therapy, implying a primary mitochondrial pathogenesis.
 tips and tricks
tips and tricks
risk factors for statin myopathy
- Advanced age
- Diabetes
- Drug–drug interactions (i.e. amiodarone, cyclosporine, colchicine)
- Female sex
- Hypothyroidism
- Liver failure
- Pre-existing muscle disorder
- Renal failure
Impaired metabolic activity involving the breakdown of fatty acids and carbohydrates represents alternative mechanisms supported by class III evidence, e.g. statins have been reported to exacerbate or trigger muscle complaints and hyperCKemia in patients who harbor single heterozygous mutations in genes known to cause metabolic myopathies (i.e. PYGM, CPTII).
Calcium dysregulation is another hypothesis regarding the pathogenesis of statin myopathy. Capacchione et al. reported a case of a 30-year-old physically fit African–American man who was started on simvastatin (20 mg daily) 1 month before developing exertional rhabdomyolysis (CK > 10 000 IU) from a benign 2.5 mile walk. Genetic screening revealed multiple polymorphic variations in calcium-handling proteins. This highlights the potential myopathic triggering effect of statins in individuals who possess a latent genetic diathesis. Patients with a history of exertional rhabdomyolysis should not be started on statins until a thorough neuromuscular work-up has been completed. Patients with a history of malignant hyperthermia and rippling muscle disease should be monitored closely for adverse effects.
Immune factors have also been postulated to play a role in the development of statin myopathy/myositis in a subset of patients. There are reports of cases with inflammatory infiltrates on muscle biopsy consistent with dermatomyositis and polymyositis; however, there are also patients who develop a myopathy in which the biopsy reveals MHC-1 upregulation but with no evidence of inflammatory infiltration – referred to as a necrotizing myopathy (NM). NMs can be idiopathic, paraneoplastic, or secondary to a connective-tissue disorder. The observation that a NM can develop after statin discontinuation suggests that previously restricted epitopes may be exposed by statin therapy through a toxic mechanism, and this may trigger a subsequent autoimmune myopathy manifesting either necrotizing or inflammatory changes.
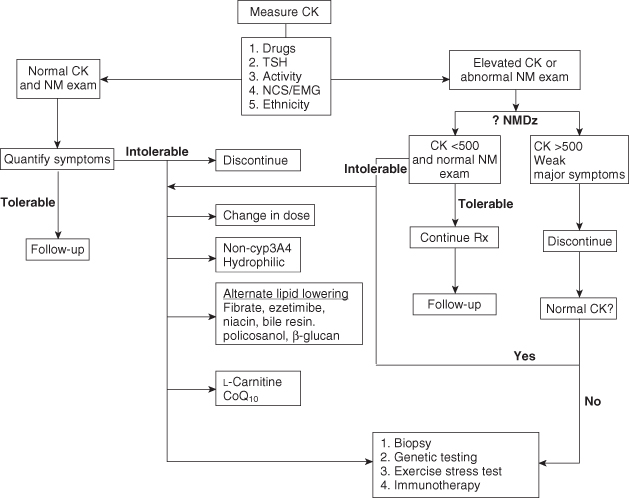
Given that numerous mechanisms may account for statin myopathy it is not surprising that a variety of neuromuscular diseases can potentially be triggered or “unmasked” by statin treatment. In certain circumstances it may be advisable to avoid statin therapy in patients with pre-existing muscle disorders and choose a bile-acid resin or low-dose fibrate as the safest alternative. An approach to inform clinical decision-making around patients suspected of statin myopathy is suggested below (Figure 4.1).
Figure 4.1. Management algorithm for statin-induced muscle disease. The box under “Measure CK” highlights five immediate considerations when assessing a patient with suspected statin myopathy. Drug interactions, hypothyroidism, exertional hyperCKemia, active denervating radiculopathy or neuropathy, and ethnic-specific CK references should be ruled out as the preliminary steps in the work-up process. CK, creatine kinase; CoQ10, coenzyme Q10; CYP, cytochrome P450; EMG, electromyography; NCS, nerve conduction studies; NM, neuromuscular; NMDz, neuromuscular disease; Rx, therapy; TSH, thyroid-stimulating hormone.
(Reproduced from Baker SK, Samjoo IA. A neuromuscular approach to statin-related myotoxicity. Can J Neurol Sci 2008;35:8–21,with permission from Canadian Journal of Neurological Sciences.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


