Felbamate
William H. Theodore
Introduction
Felbamate (2-phenyl-1, 3-propanediol dicarbamate) (FBM), structurally unrelated to any other antiepileptic drug (AED) in current clinical use, is particularly interesting because its pharmacologic activity in animal models suggests a spectrum broader than that of most other AEDs, indicating the potential for clinical efficacy in patients with generalized as well as localization-related epilepsies.68 Moreover, it is one of the few nonsedating agents available. Unfortunately, it is associated with a high risk for hematologic and hepatic toxicity.
Chemical structure
FBM is related structurally to the antianxiety drug meprobamate (Fig. 1A). Its molecular weight is 238.24, and the factor to convert from micrograms (μg) per milliliter to micromoles (mmol) per liter is 4.2. It is relatively insoluble in water or ethanol, but it penetrates the central nervous system readily.11,59
FluoroFBM (FFBM, 2-phenyl-2-fluoro-1, 3 propanediol dicarbamate), currently in preclinical development, is an analog designed to have clinical efficacy similar to FBM without the serious adverse effects of the latter.4 FFBM has a fluorine substituted for hydrogen in the 2-position of the propanediol moiety (Fig. 1B). This substitution appears to prevent the production of the reactive toxic metabolite of FBM, atropaldehyde (ATPAL, or 2-phenylpropenal). If FFBM does prove to have similar efficacy and dose-related toxicity, but no severe systemic side effects, it may become an extremely useful AED; there are still only a few without cognitive side effects.
Methods for Determination in Body Fluids
High-performance liquid chromatography (HPLC) with spectrophotometric detection is the standard method for FBM measurement; multiple AEDs can be measured simultaneously.9 Gas-liquid chromatography and HPLC provide comparable results.23 Capillary electrophoresis has been proposed as a more rapid alternative.72 Interlaboratory variation does not depend on the method used; it may be greater for AEDs like FBM that are measured less frequently, and laboratory participation in an external quality control program is essential.89
Pharmacology
Activity in Experimental Models of Seizures/Epilepsy
FBM is active in maximal electroshock, pentylenetetrazole, bicuculline, and picrotoxin seizure models in experimental animals, suggesting a broad spectrum of activity.58 Its protective index (TD50/ED50) in the rat maximal electroshock model is 63 (compared with 100 for phenytoin), and in the pentylenetetrazole model it is 12 (vs. 1.6 for valproic acid).59 No pharmacodynamic evidence of tolerance to FBM was found in 15-day studies in rats and mice.76 FBM increased the threshold for focal seizures and reduced seizure severity, duration, and afterdischarge duration at doses that produce no adverse behavioral effects in amygdala-kindled rats.92 FBM is not protective against bupropion-induced seizures.84 FBM in doses of 100 to 500 mmol had no effect on soman-induced burst frequency, but decreased duration.26
Both FBM and its congener fluoroFBM are effective in the perforant path stimulation model of status epilepticus, even in late stages, and may have some antiepileptogenic effects.49,50 Some studies have suggested pharmacodynamic synergism between lamotrigine and FBM in activity against maximal electroshock, 4-aminopyridine, and pentylenetetrazole-induced seizures.12,48 Interestingly, lamotrigine but not levetiracetam showed additive toxicity to FBM in mice.46,47
Mechanisms of Action
In cultured hippocampal neurons, FBM has an unusual pattern of activity, blocking currents evoked by N-methyl-D-aspartate (NMDA) but facilitating γ-aminobutyric acid (GABA)ergic responses.64,82,90 The pattern of interaction with GABAA receptors suggests relatively weak, barbiturate-like, modulatory (rather than direct agonist) activity.64 Strychnine (0.25–0.5 mg/kg) and picrotoxin (3 mg/kg) impaired the protective activity of FBM against maximal electroshock (MES), suggesting that GABAergic inhibition and strychnine-insensitive glycine receptor–mediated events may contribute to anticonvulsant activity.75
Proposed mechanisms and sites for the NMDA receptor interaction include competitive antagonism at the glycine site, open channel blocking or, most likely, noncompetitive allosteric channel effect.51 FBM does not appear to affect inhibitory glycine receptor currents in hippocampal neurons.35 These features may explain both the AED spectrum of FBM and its stimulant-like side effects at clinical doses. FBM has neuroprotectant effects that could be mediated by NMDA receptor interaction.52 Several pharmacologic features of FBM may account for its clinical properties. At therapeutic concentrations (50–300 mmol) FBM may bind to resting NMDA channels in the absence of glutamatergic ligands and decrease subsequent NMDA currents by stabilization of desensitized channels, thus inhibiting seizure discharges but having less effect on normal activity.44 NMDA subunit specificity, with selectivity for NR1a/NR2B subunits, could have a similar effect.27,40
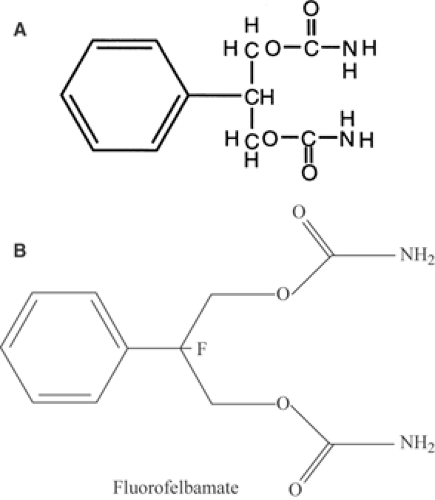 FIGURE 1. A: Structure of FBM. Although it resembles meprobamate, it has no anxiolytic effects. B: Structure of fluorofelbamate. |
Some studies suggest that sodium (Na) channel blockade may contribute to antiseizure activity. FBM inhibited voltage-dependent Na+ currents when α-subunits from rat and human brain were expressed in Xenopus oocytes, but only when
perfused on the intracellular side of the membrane.77 FBM also blocked sustained repetitive firing in mouse spinal cord neurons.90
perfused on the intracellular side of the membrane.77 FBM also blocked sustained repetitive firing in mouse spinal cord neurons.90
Activity in Other Models
In rats, FBM (100 and 300 mg/kg) attenuated overall naloxone-precipitated opiate withdrawal severity in a dose-related manner and reduced occurrences of chews, teeth chatters, and penile grooming, possibly due to glycine antagonist activity.42 A similar mechanism may account for FBM’s efficacy against D2 (haloperidol) but not D1 receptor–mediated akinesia, a pattern similar to other NMDA receptor complex glycine site antagonists.43
Toxicity Models
Early studies had shown only relatively nonspecific effects, such as decreased body weight and food consumption in mice, rats, and dogs, and increased liver weight without associated histopathological changes.54 An increased incidence of hepatic cell adenoma and of interstitial cell tumors of the testes in rats also occurred.53
Recognition of FBM’s idiosyncratic toxicity has triggered a search for possible mechanisms. Most studies have suggested that reactive intermediates, such as atropaldehyde (2-phenyl-propenal) an unsaturated aldehyde, may be the most important metabolites for hepatic and hematological toxicity.14,33,34 2-Phenylpropenal irreversibly inhibits GSTM1-1, the form of glutathione-S-transferase in liver.15,16
Glutathione might protect against reactive intermediate formation, although some toxicity mechanisms may be redox-independent.29,60 Interestingly, the level of uridine diphosphoglucuronosyltransferase activity had no effect on the metabolic pathway in rats.14 In human liver preparations, FFBM does not form reactive intermediates.4
Clinical pharmacokinetics
Formulations
FBM is available in 400- and 600-mg scored tablets, and in a suspension containing 600 mg/5 mL. Because there is no difference in bioavailability, the formulations should be interchangeable.
Absorption
Plasma Protein Binding and Distribution
Plasma concentration, area under the curve (AUC), and maximal concentration (Cmax) increase linearly with dose.58 The apparent volume of distribution, about 0.8 L/kg, is the same after a single dose or continued administration. FBM is relatively lipid-soluble and readily crosses the blood–brain barrier.11 P-glycoprotein but not ABCC2/MRP2-mediated efflux may limit FBM brain access in rats.61,62 FBM is 20% to 25% reversibly bound to plasma protein, mainly albumin. FBM does not show preferential accumulation in any peripheral organ or specific brain region. Pharmacokinetic parameters are stable during continued administration.11
Metabolism and Elimination
FBM is metabolized by the cytochrome P450 system, which probably accounts for its interactions with other AEDs.59 The terminal elimination half-life ranges from 13 to 23 hours. About 40% to 50% of the absorbed dose appears unchanged in the urine. Metabolites include p-hydroxyphenyl FBM, 2-hydroxy FBM, monocarbamate derivatives, and polar compounds (including conjugates), none of which has significant antiepileptic activity; these make up about 15% of plasma radioactivity when labeled FBM is given.58 Children may metabolize the drug more quickly than adults. Beagle pups had decreased bioavailability but unchanged absorption and volume of distribution compared with adult beagles, suggesting faster elimination.1 In humans, clearance is 40% higher in children than adults, and half-life approximately is 15 hours.5,7 However, there do not seem to be any sex-related differences. FBM blood levels are stable during long-term treatment, with no evidence for autoinduction of metabolism.17,68
Drug Interactions
When FBM is used, drug interactions may be an important factor. FBM reduces blood carbamazepine levels by about 20%, whereas levels of the active metabolite, carbamazepine epoxide, are increased by 25% to 50%.2,19,22,82,86
Several investigators22,45,85,91 have reported that FBM increases phenytoin, valproic acid, and phenobarbital levels. The doses of these drugs may need to be reduced by 10% to 30%
to maintain constant levels when FBM is given concurrently, although the effect varies from patient to patient. For phenytoin, the effect on blood levels may be related to increasing FBM doses.71 FBM increases conversion of clobazam to its active metabolite N-desmethyl-clobazam (N-CLB), apparently to a greater degree than does phenytoin or carbamazepine.10 Because the metabolite appears to be more potent than the parent drug, this effect could increase both efficacy and toxicity. FBM does not appear to have a clinically significant interaction with clonazepam, lamotrigine, or vigabatrin.79,87
to maintain constant levels when FBM is given concurrently, although the effect varies from patient to patient. For phenytoin, the effect on blood levels may be related to increasing FBM doses.71 FBM increases conversion of clobazam to its active metabolite N-desmethyl-clobazam (N-CLB), apparently to a greater degree than does phenytoin or carbamazepine.10 Because the metabolite appears to be more potent than the parent drug, this effect could increase both efficacy and toxicity. FBM does not appear to have a clinically significant interaction with clonazepam, lamotrigine, or vigabatrin.79,87
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






