Figure 54.1. Structure of felbamate.
Antiepileptic Profile in Animals
FBM displayed high protective indices (toxic dose50/effective dose50) against the tonic phase of both maximal electroshock seizures (MES) and subcutaneous pentylenetetrazol-induced seizures in rodents (1). It is effective in amygdala-kindled, phenytoin (PHT)-resistant rats (11).
Mechanisms of Action
FBM binds to open ion channels of the N-methyl-d-aspartate (NMDA) subtype of the glutamate receptor (12) and may also modulate NMDA receptor function by binding to an associated strychnine-insensitive glycine receptor (13). These actions inhibit sodium and calcium excitatory currents. The NMDA effect of FBM is unique among available AEDs. Binding is more specific to receptors containing the N2RB subunit of the glutamate receptor (14,15). Subtype specificity may account for the lack of serious neurobehavioral complications and impairment of learning, which might be expected of NMDA receptor blockers (16).
FBM may also affect non–NMDA-type glutamate channels (17), voltage-gated sodium channels (17), high-threshold voltage-sensitive calcium currents (18), and GABA-mediated chloride current inhibition (19).
The antiglutamatergic mechanism may imply neuroprotective effects: FBM reduced neuronal damage in a rat model of hypoxia–ischemia (20), protected CA1 hippocampal neurons from apoptosis in a gerbil ischemia model (21), and exhibited neuroprotective effects in a rat model of status epilepticus (22).
ABSORPTION, DISTRIBUTION, AND METABOLISM
FBM is well absorbed; more than 90% of 14C-labeled FBM, or its metabolites, is recovered in urine and feces after oral administration (23). The rate and extent of absorption are not affected by food or antacids (24). Protein binding in human plasma is low, 22% to 25% (23). FBM readily crosses the blood–brain barrier (25).
Of the absorbed FBM dose, 30% to 50% is excreted in the urine unchanged (23). The remainder—more if there is renal insufficiency—is metabolized by the liver, utilizing CYP3A4 and CYP2E1 isozymes (23). Clearance in children is up to 40% higher in comparison to adults (26) but does not decrease significantly in the elderly (27).
In humans, FBM exhibits linear first-order kinetics over a dose range of 1200 to 6000 mg/day (28). Peak plasma concentration is reached 3 hours after an oral dose (23). Monotherapy with FBM 3600 mg/day produced a mean trough plasma level of 78.4 μg/mL (range, 23.7 to 136.6 μg/mL) in one study (7) and a mean (±standard deviation) level of 65 (±23) μg/mL after 112 days in another (8).
The mean terminal elimination half-life of 20 hours (range, 13 to 23 hours) in monotherapy decreases to 13 to 14 hours in the presence of phenytoin (PHT) or carbamazepine (CBZ) (3). The apparent volume of distribution is 0.8 L/kg (3,29). Steady-state plasma levels are achieved in 4 days (29).
EFFICACY
FBM is approved for use in the United States as either adjunctive therapy or monotherapy for patients older than 14 years of age with partial seizures, with or without generalization, and as adjunctive therapy for patients of any age with Lennox–Gastaut syndrome and its component seizure types (29).
Partial-Onset Seizures
Early clinical studies of FBM employed standard adjunctive trial designs. Adding FBM to CBZ (4,5) or to PHT (4) in outpatient trials produced modest reductions in seizure frequency. An adjunctive therapy trial among patients in an inpatient seizure monitoring unit produced a more encouraging result (6). The primary end point was time to occurrence of a fourth seizure or 29 days, whichever came first. Of patients randomized to adjunctive placebo, 88% had a fourth seizure, compared to 46% taking adjunctive FBM (P = 0.03) (6). Nevertheless, the authors of a Cochrane review concluded that there is “no reliable evidence to support the use of FBM as an add-on therapy in patients with refractory partial-onset epilepsy” (30). A flaw in this conclusion is that CBZ and PHT strongly induce the metabolism of FBM, and it is possible that suboptimal FBM concentrations were achieved in those three trials (4–6). The efficacy of FBM when added to newer non–enzyme-inducing drugs has not been tested in humans, although a synergistic effect with levetiracetam was shown in a mouse MES model (31).
In 1988, new monotherapy designs for AED trials were proposed in a workshop sponsored by the NIH (32). Clinical investigators of FBM were the first to use these designs. The presurgical design was repeated as a monotherapy trial, and results suggested good efficacy (33). In two outpatient monotherapy trials, standard therapy was withdrawn over 28 days, while FBM 3600 mg/day or valproate (VPA) 15 mg/kg/day was substituted (7,8). The VPA control requires explanation: it was a compromise between a placebo control, considered unsafe, and a flexible dose active control, which could have reduced the chance of detecting a difference (34). This design has been criticized as violating the concept of equipoise, but 15 mg/kg/day is the recommended starting dose for VPA. Therefore, patients were randomized to a drug with unknown efficacy—FBM—or to the recommended initial dose of a drug with proven efficacy for their seizure type—VPA. The end point, “escape” (treatment failure), was defined individually for each patient according to predetermined criteria, including doubling of seizure frequency during any 2-day or 30-day period compared to the pretreatment baseline. Patients were removed from the trial at once if one of the end points were met. Patients taking low-dose VPA met escape criteria more often than did FBM-treated patients (86% vs. 14%, respectively, of 42 patients in the single-center study (7); 78% vs. 40%, respectively, of 95 patients in the multicenter study (8). Experience with partial-onset seizures in children is limited.
Lennox–Gastaut Syndrome and Other Seizure Types
FBM 45 mg/kg/day was used as adjunctive therapy, most often as an adjunct to valproate, in a multicenter, double-blind, controlled trial of 73 patients with a diagnosis of Lennox–Gastaut syndrome (35). Atonic seizures (drop attacks) were reduced by 34% and all seizures by 19%, versus a 9% decrease and a 4% increase, respectively, with placebo. During a 12-month, open-label follow-up, seizure frequency decreased by 50% with adjunctive FBM, compared to 15% with adjunctive placebo (36).
Infantile spasms may respond (37). Among 38 children with severe intractable epilepsies (22 Lennox–Gastaut syndrome, 6 Doose syndrome, and 10 other syndromes), FBM rendered 16% seizure free and 63% experienced a reduction of >50% in seizure frequency (38). Children younger than 4 years of age with various seizure types have sometimes responded well (39).
DRUG INTERACTIONS
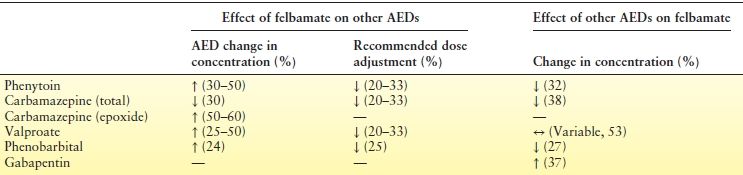
Skillful use of FBM requires knowledge of several drug interactions. Primary references for these interactions are available from a review article (40). Clinically significant interactions are summarized in Table 54.1. FBM often acts as an enzyme inhibitor, especially of the cytochrome P450 2C19 isozyme. FBM therefore increases serum levels of many other drugs, including phenobarbital, PHT, and warfarin. FBM raises VPA levels by inhibiting its beta oxidation. Typical clinical toxicities of concomitant drugs, such as ataxia with PHT, may occur when FBM is added. The FBM/CBZ interaction is unusual: when FBM is added to CBZ, levels of CBZ decrease by 20% to 30%, but CBZ epoxide (CBZ-E) levels increase by 50% to 60%. The increase in CBZ-E can cause clinical toxicity such as dizziness, diplopia, or headache.
Table 54.1 Interactions of Felbamate with Other AEDS
AEDs, antiepileptic drugs.
In contrast, enzyme-inducing drugs may lower effective FBM serum levels by enhancing its metabolism via CYP2E1 and CYP3A4 pathways. This effect is pronounced for PB, CBZ, and PHT, which lower FBM levels by 27%, 32%, and 38%, respectively (40).
Oddly, CYP3A4 inhibitors such as erythromycin have little effect on FBM metabolism. VPA, however, does inhibit the metabolism of FBM, though the extent is not clear from the limited data. A nonmetabolic effect of gabapentin (GPN) in raising FBM serum levels is thought to be due to an interaction at the renal excretion level: GPN can increase FBM elimination half-life by 46% (40).
ADVERSE EFFECTS
Common Adverse Effects
The overall dropout rate attributed to adverse effects in clinical trials was 12% (29), a figure not dissimilar to that seen in clinical trials of other new AEDs. Gastrointestinal disturbances, headache, anorexia, and insomnia are common (7,8,29). Weight loss is most likely over the first year of use, then may stabilize (29). Dizziness, diplopia, and ataxia were more common in adjunctive therapy than with monotherapy (4,5,7,8) and thus may have been related to pharmacokinetic elevations in PHT and CBZ-E levels.
Monotherapy is better tolerated. Among 366 adults receiving monotherapy, 4.1% experienced nausea, 3.6% insomnia, 3% anorexia, 2% to 5% dizziness, and 2% weight loss (7,8,33). Administering FBM in three daily doses after meals may reduce stomachache.
FBM has a stimulant effect in many patients. This is a major advantage in comparison to other AEDs, but it may be associated with insomnia, irritability, and behavioral changes. Giving the largest dose in the morning may help insomnia. At 3% to 4%, the incidence of rash was no higher than placebo in clinical trials (29). Two children experienced involuntary dyskinetic movements (41). There is one report of a kidney stone (42).
Dose-Limiting Effects
Doses in clinical trials were limited to 3600 mg/day for adults and 45 mg/kg/day for children. Some patients cannot achieve these doses without side effects, especially in adjunctive therapy. Higher doses are sometimes well tolerated: among 50 patients stabilized on 3600 mg/day whose dose was raised to 4200 to 7200 mg/day (mean 5412 mg/day, mean serum concentration 110 mg/L), 32% developed new or increased side effects, but only 15% required dose reductions (43). The most common dose-limiting effects in this group were dizziness, ataxia, and nausea.
Aplastic Anemia
FBM can cause aplastic anemia. In 1994, 33 cases were reported to the FDA (9,44,45). Another case was reported in 2000 and a questionable one in 2007 (29). There have been 14 fatalities. Detailed review of the first 31 cases according to International Agranulocytosis and Aplastic Anemia Study criteria revealed that 23 (74%) met criteria for a diagnosis of aplastic anemia (44). Six others had preexisting blood dyscrasias or systemic lupus erythematosus. Of the 23 confirmed cases, FBM was implicated as the most likely cause in 14; others had other plausible causes, usually other medications known to cause aplastic anemia.
Based on a 1997 estimate of 110,000 patients exposed, this yields a most probable incidence of 127 per million (1/8000 cases), compared with a population rate of 2 per million per year, with a worse-case incidence of 300 per million (44). By comparison, estimates for CBZ range from 5 to 39 per million per year (44). All FBM-related aplastic anemia cases were diagnosed within 1 year of starting the drug, two-thirds within 6 months (45). This suggests that the risk drops substantially after 1 year.
Patients developing aplastic anemia were more likely to have histories of blood dyscrasias, especially cytopenia, autoimmune disorders, and rashes, or significant toxicities with previous drugs (45,46). It seems best to avoid FBM in such patients. FBM may be safer for children; only one child, a postpubescent 14-year-old, has been affected (29).
Liver Failure
Among patients taking FBM for 25 to 959 days, 18 reported cases of liver failure resulted in 9 fatalities (45,46). Of these 18, 8 cases may have been caused by other factor: 5 with status epilepticus and 1 case each of hepatitis A, acetaminophen poisoning, and severe hypotension. Using population exposure estimates, this implies a risk of about 1 per 10,000 patient exposures.
Mechanisms of Toxicity
The mechanism by which FBM causes bone marrow and liver toxicity is unknown, but the formation of a toxic metabolite that triggers an immune reaction is suspected. The second step in FBM metabolism is formation of 3-carbamoyl-2-phenylpropionaldehyde (CBMA) (47). CBMA is then metabolized by three competing pathways, one of which leads to the formation of 2-phenylpropenal, also known as atropaldehyde (47). Atropaldehyde is cytotoxic and immunogenic, and it may be that individuals who form more of this compound on a genetic basis are more at risk. The aldehyde undergoes beta-elimination to 2-phenylpropenal, another known toxic compound (48). Since atropaldehyde is detoxified by glutathione, and glutathione stores are depleted by acetaminophen, it seems prudent to advise patients on FBM therapy not to take acetaminophen, although this notion is theoretical. There may be other mechanisms for blood toxicity. Both FBM and metabolites can cause apoptosis of bone marrow progenitor cells in vitro (49). Fluorofelbamate, a potent antiepileptic compound that is not metabolized to atropaldehyde, has been proposed as a safer alternative to FBM (50), and other carbamate derivatives of FBM show promise as anticonvulsant agents (51). However, it will be difficult to conduct human trials of these drugs without very thorough reassurance from animal safety studies.
CLINICAL USE
Patient Selection
Because of the low but definite risk of serious blood or liver reactions, FBM should not be used as initial therapy or when an effective alternative can be found. Patients with focal seizures refractory to several drugs, especially with both severe epilepsy and drug sedative side effects, may be considered for treatment. A Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society have formulated practice guidelines (52) (Table 54.2). All patients or their caretakers must be able to report side effects reliably, comply with blood testing, and understand potential risks and benefits.
Table 54.2 Recommendations for Use of Felbamate

Adapted from French J, Smith M, Faught E, et al. Practice advisory: the use of felbamate in the treatment of patients with intractable epilepsy. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 1999;52:1540–1546, with permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








