The Pulsante™ microstimulator

The Pulsante™ system is comprised of a handheld remote control which inductively powers the implanted microstimulator
5.3.1 Planning
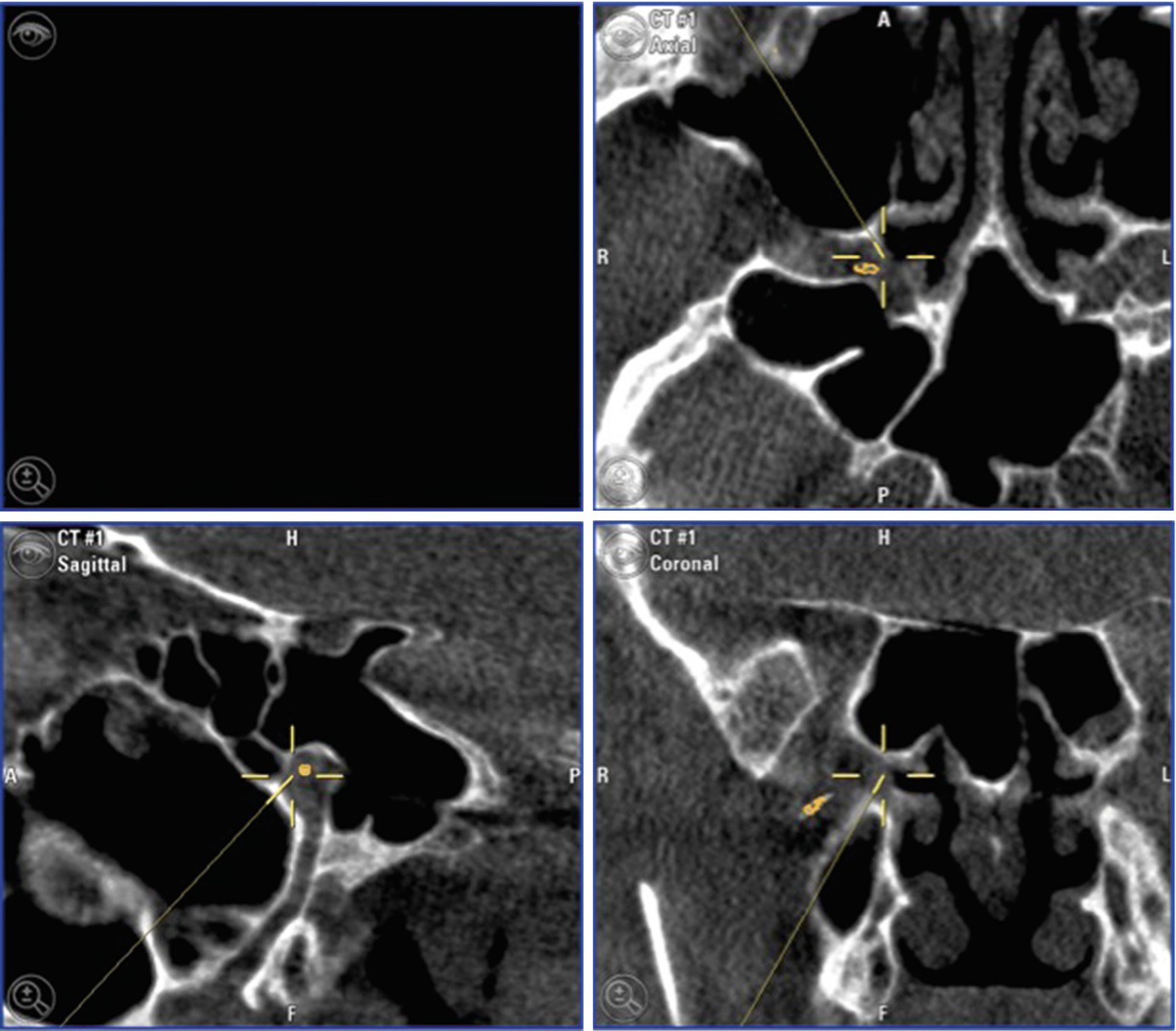
Preoperative planning of the surgical implantation is important, due to the various differences in the facial anatomy and especially in the PPF. This affects not only between different patients, but also within one patient, as the left side may be different from the right [18], [10].
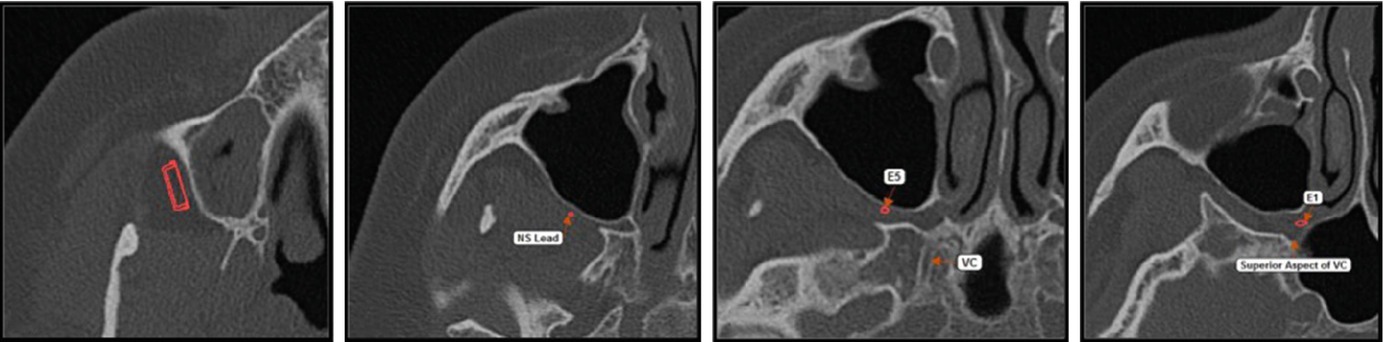
In all patients, the presence of dental pathology should be excluded prior to implantation as this may result in postsurgical infection of the implant site. To address these issues, prior to surgery, a panoramic view, dental cone beam computed tomography (CBCT), or computed tomography scans (CT) of the oral cavity an the maxillary sinus should be performed [19]. Interpretation and description of the radiological investigations should focus on signs of osteopathology including infection, pericoronitis, and impacted wisdom teeth as well as the morphology of the pterygomaxillary fissure. The latter bears importance as the lead diameter of the microstimulator is 1 mm. A minimum width of the fissure of 1.2 mm is preferable.
A unique challenge in CH is constituted by patients who have been treated for many years with pulse therapy of corticosteroids as this may lead to osteoporosis, increasing the risk of perioperative posterior maxillary wall fracture [10]. These, as well as the described radiological findings, weigh in on the final confirmation of patient eligibility at the surgeon’s discretion. Thus far, in the published studies, no patients have been rejected on the basis of surgical ineligibility but some have had preoperative dental procedures. On the establishment of surgical eligibility, the manufacturer performs 3D modeling and makes a recommendation for stimulator size—short (3.6 cm), medium (4.4 cm), long (5.2 cm), and extra-long (6.0 cm). As power and control is provided by the RC, these recommendations also figure the resulting depth of the microstimulator housing as this is essential for a functioning connection.
5.3.2 Surgery
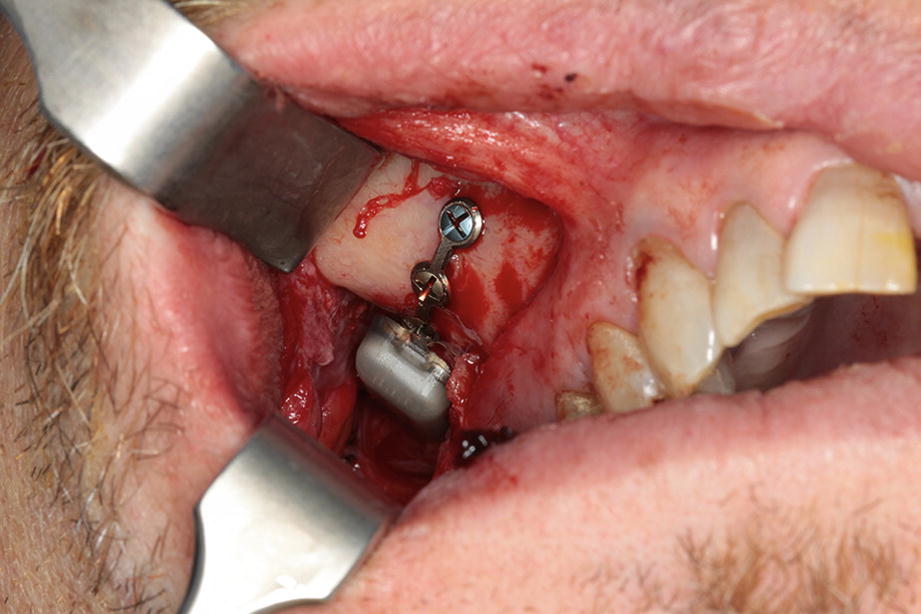
Upper gingival incision showing the fixated SPG-microstimulator with the osteosynthesis plate fixated on the lateral wall of the maxilla

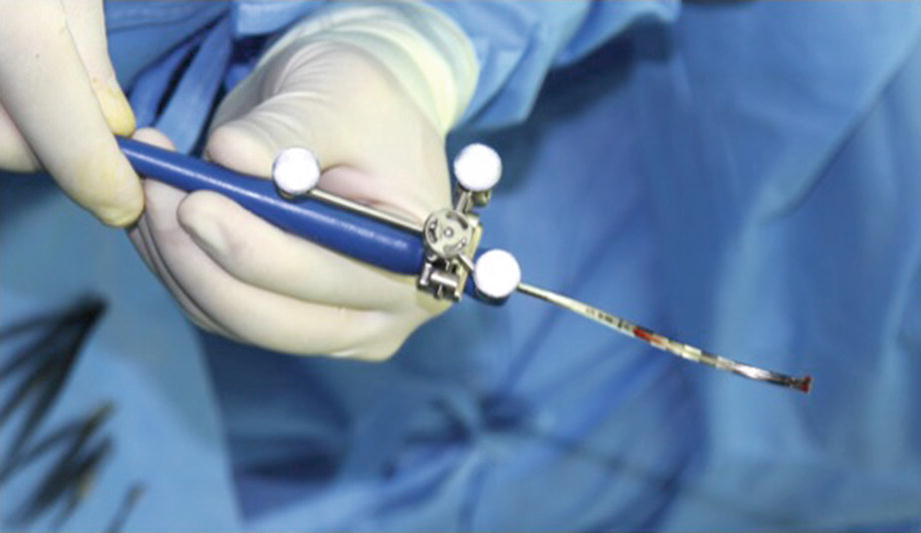
(a) Surgical Instrument (SI-100). (b) Surgical Instrument (SI-110). (c) Surgical Instrument (SI-120)
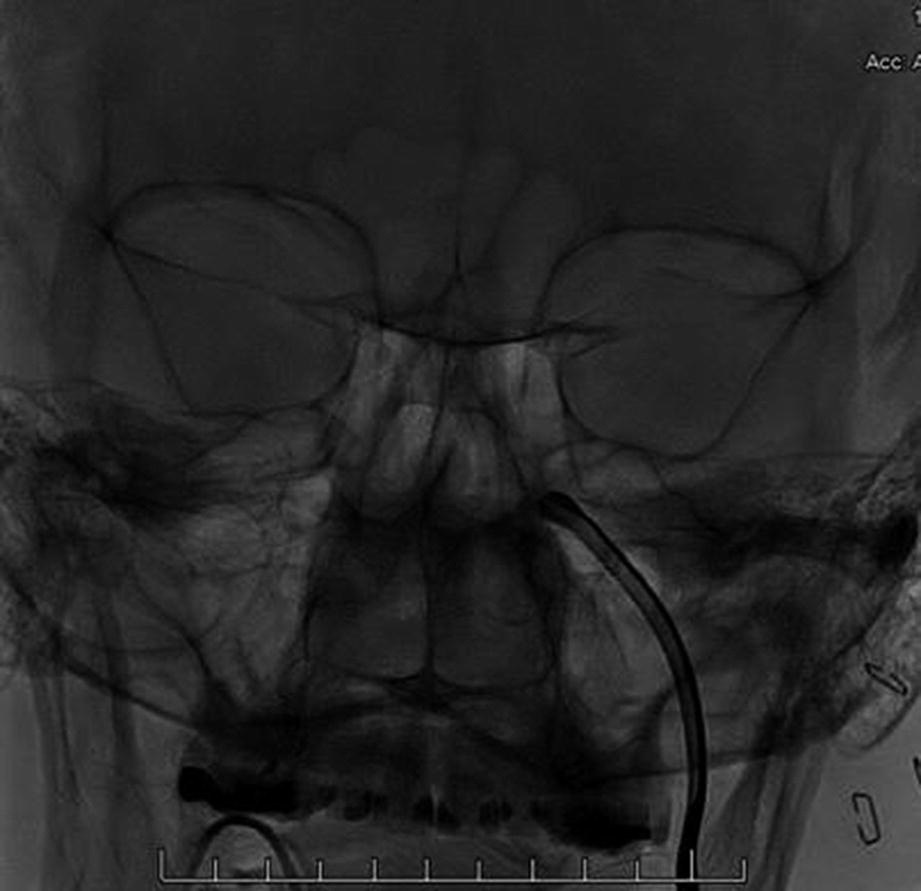
Intraoperative fluoroscopy (coronal view) showing the inserted instrument SI-100 inside the left pterygopalatine fossa (PPF)
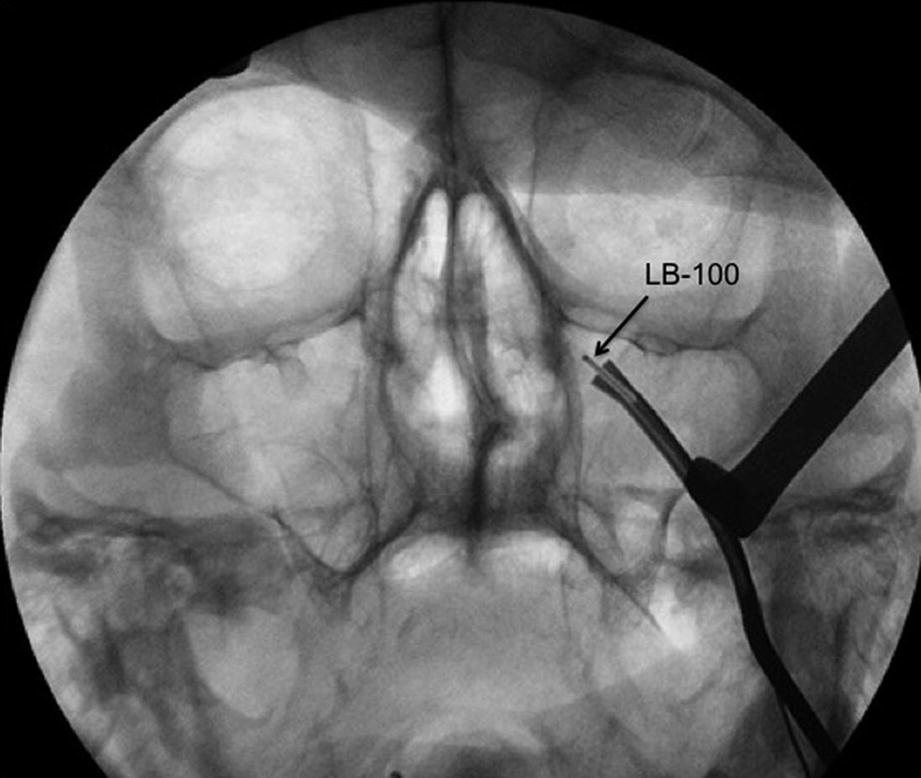
Intraoperative fluoroscopy (coronal view) showing the inserted instrument SI-110 with the lead blank (LB-100) inserted via the SI-110 into the left pterygopalatine fossa (PPF)
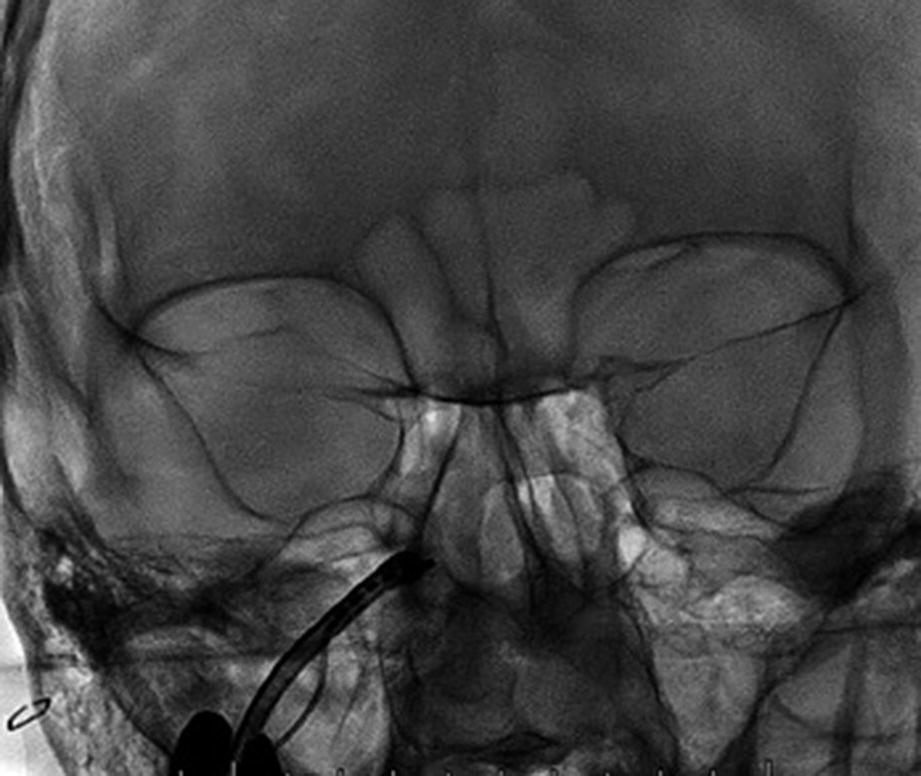
Intraoperative fluoroscopy (coronal view) showing the SI-120 inserted to the rigth PPF
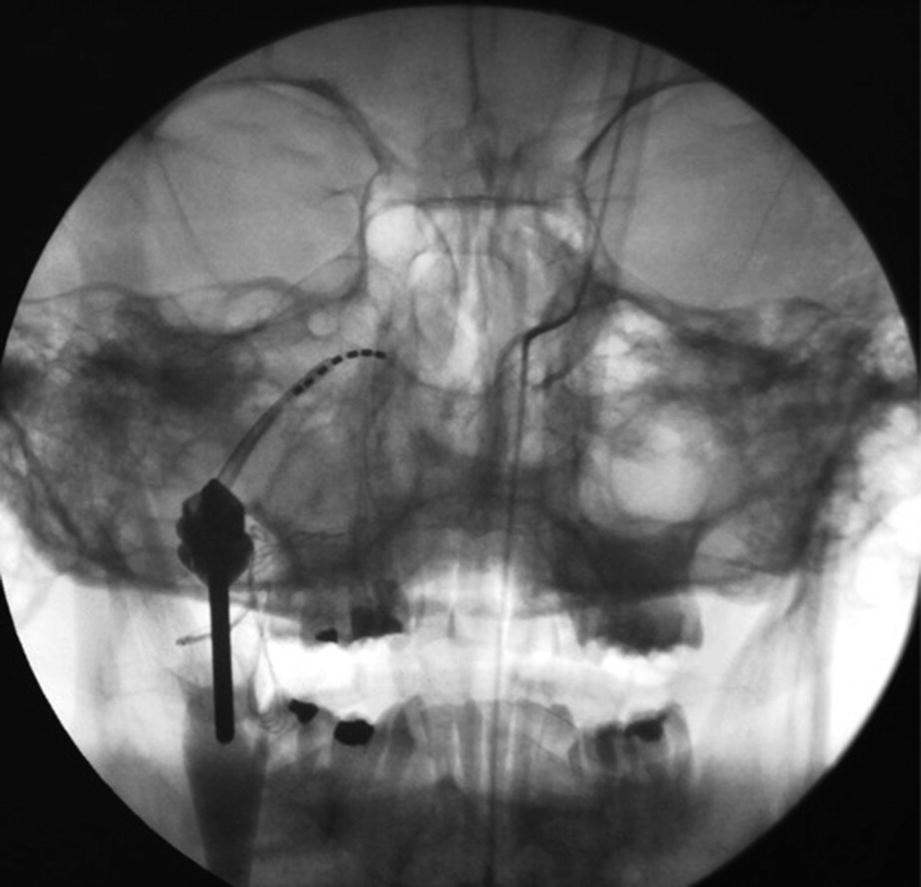
Intraoperative fluoroscopy (coronal view) showing the SI-120 with the inserted SPG-micorstimulator inserted to the rigth PPF
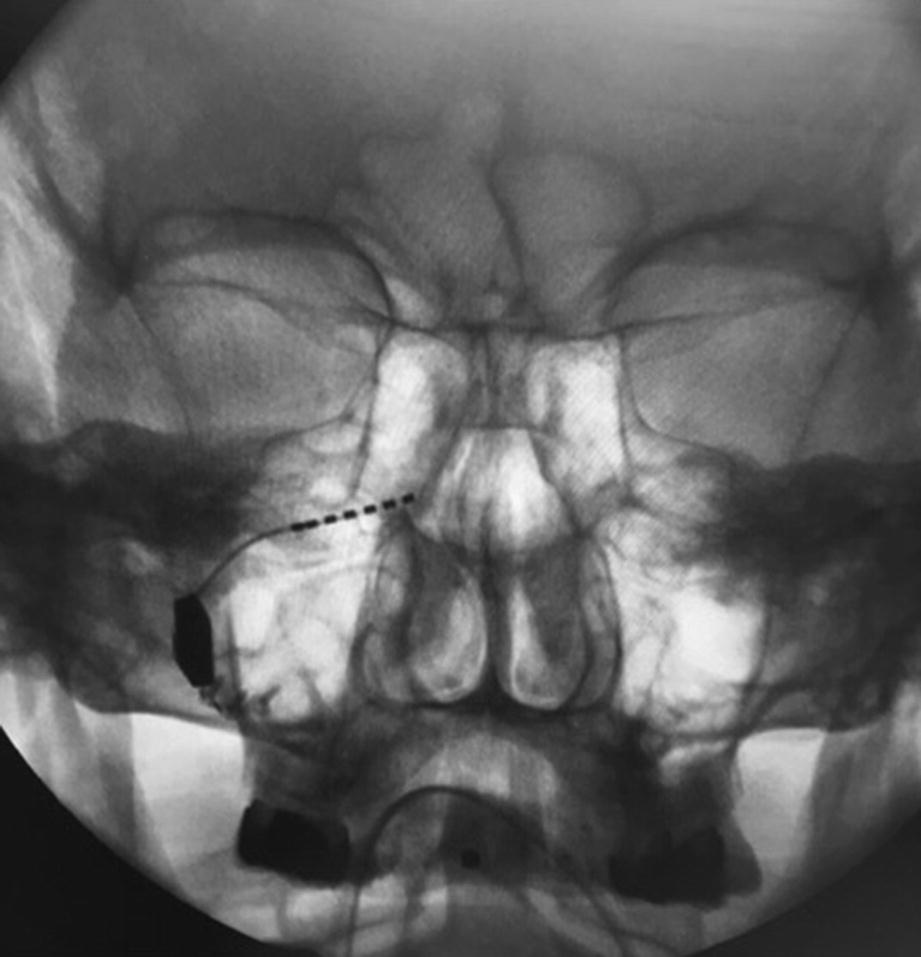
Intraoperative fluoroscopy (coronal view) showing the inserted SPG-micorstimulator inside the rigth PPF, fixated with 3 osteosynthesis screws
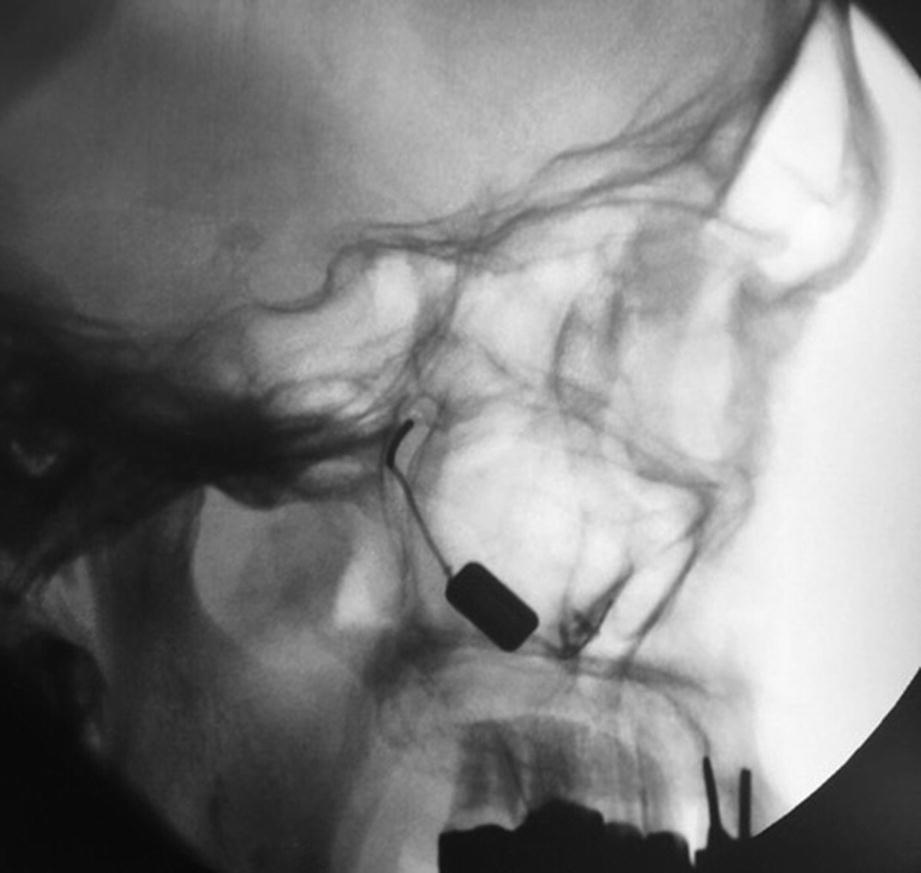
Intraoperative fluoroscopy (sagittal view) showing the inserted SPG-micorstimulator inside the rigth PPF, fixated with 3 osteosynthesis screws
Microstimulator function is checked prior to insertion and closing suturing. After surgery, correct placement of the device is assured by intraoperative 3D-CT if possible, or a postoperative 3D-CT. If necessary, placement may be revised. The procedure is considered minimally invasive—comparable in extent to that associated with wisdom tooth extractions or dental implants procedures. The average duration of the surgery for the first 99 patients was 80 min (range 25–175) [19].
5.3.3 Surgical Intraoperative Navigation
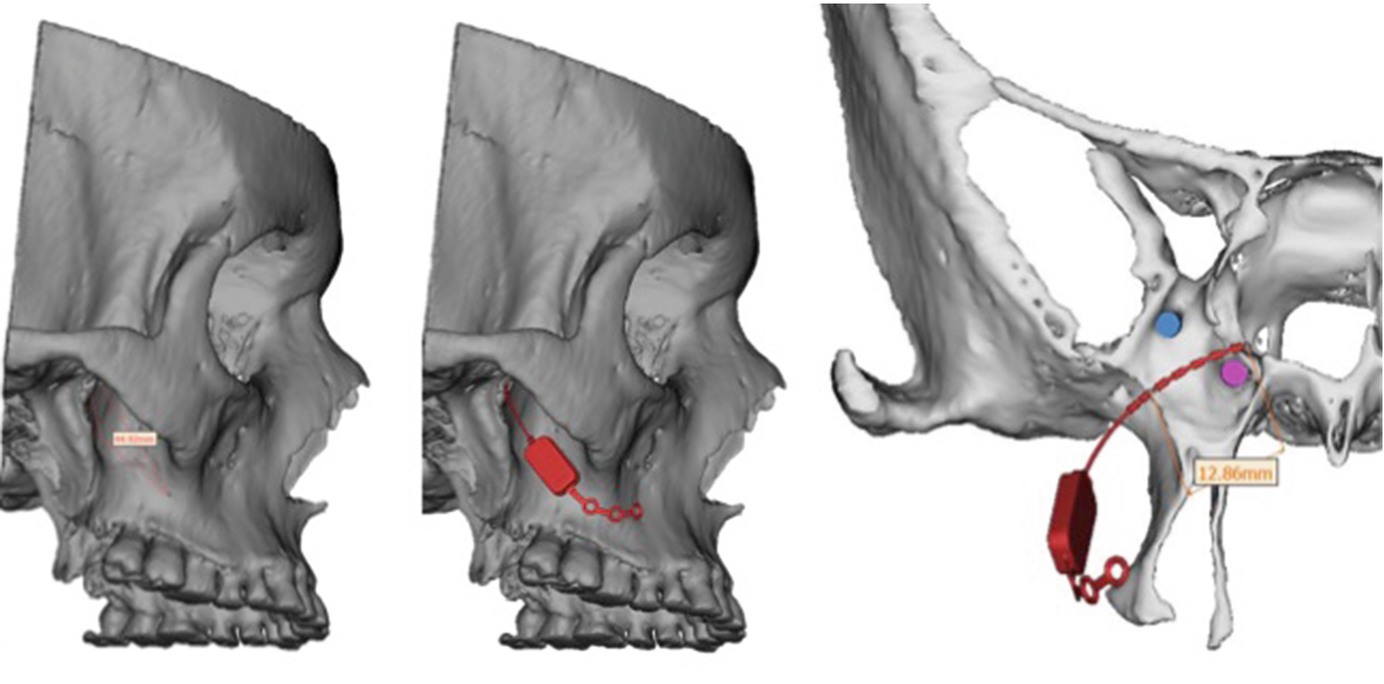
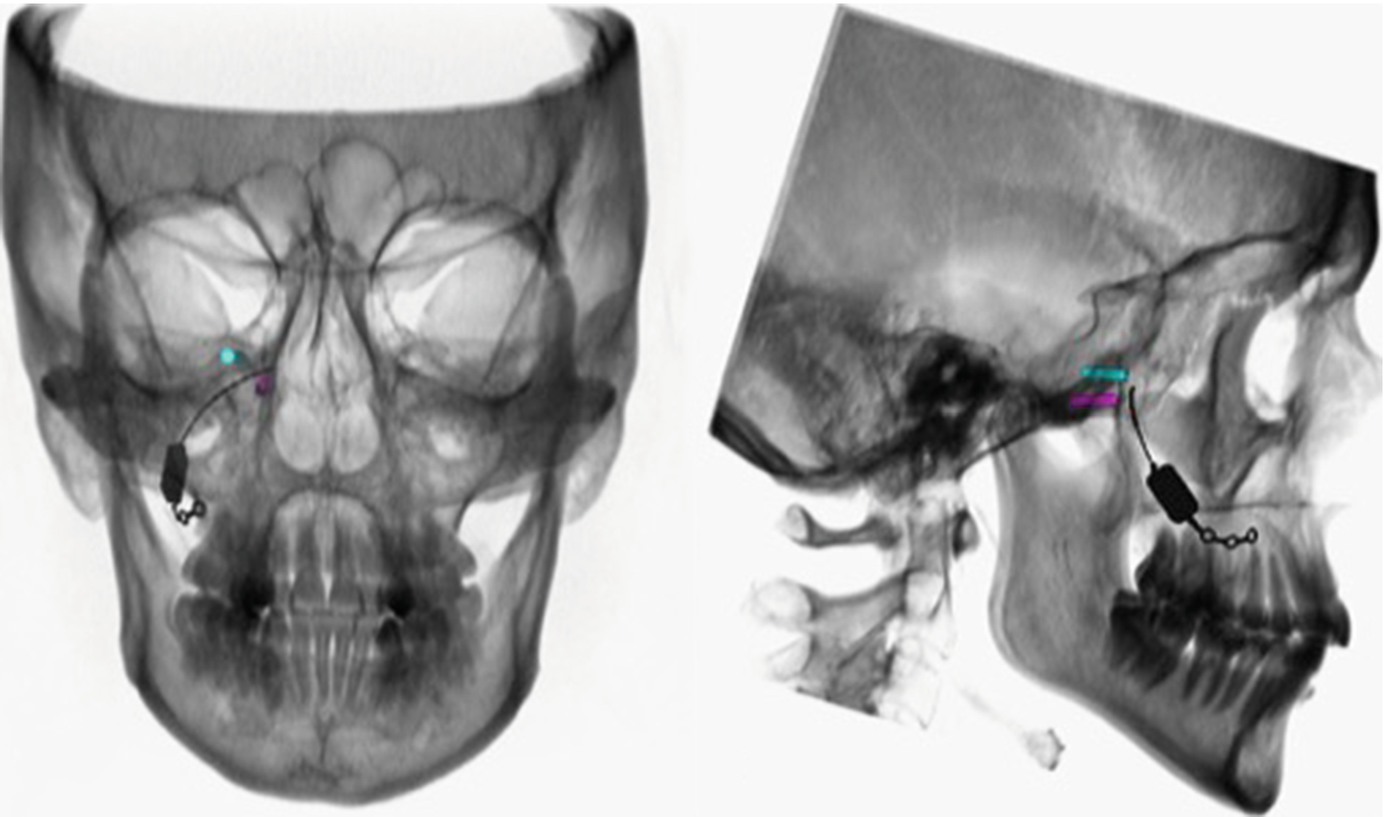
Postoperative 3D reconstruction of the inserted SPG-microstimulator showing the postition inside the PPF between the Foramen rotundum and the Vidian canal
Reconstruction of the CT-scans as DRR for intraoperative comparison
3D-CBCT data with the virtual treatment plan showing the ideal positioning of the SPG-microstimulator
Intraoperative Navigation (Brainlab system) showing the marker spheres of the used system which are attached to the patients’ calvarian bone for skull reference array (SRA)
Further marker spheres are attached to the surgical instruments (SI-100 and SI-110) as demonstrated here
The system displays the actual position during insertion of the instruments inside the PPF
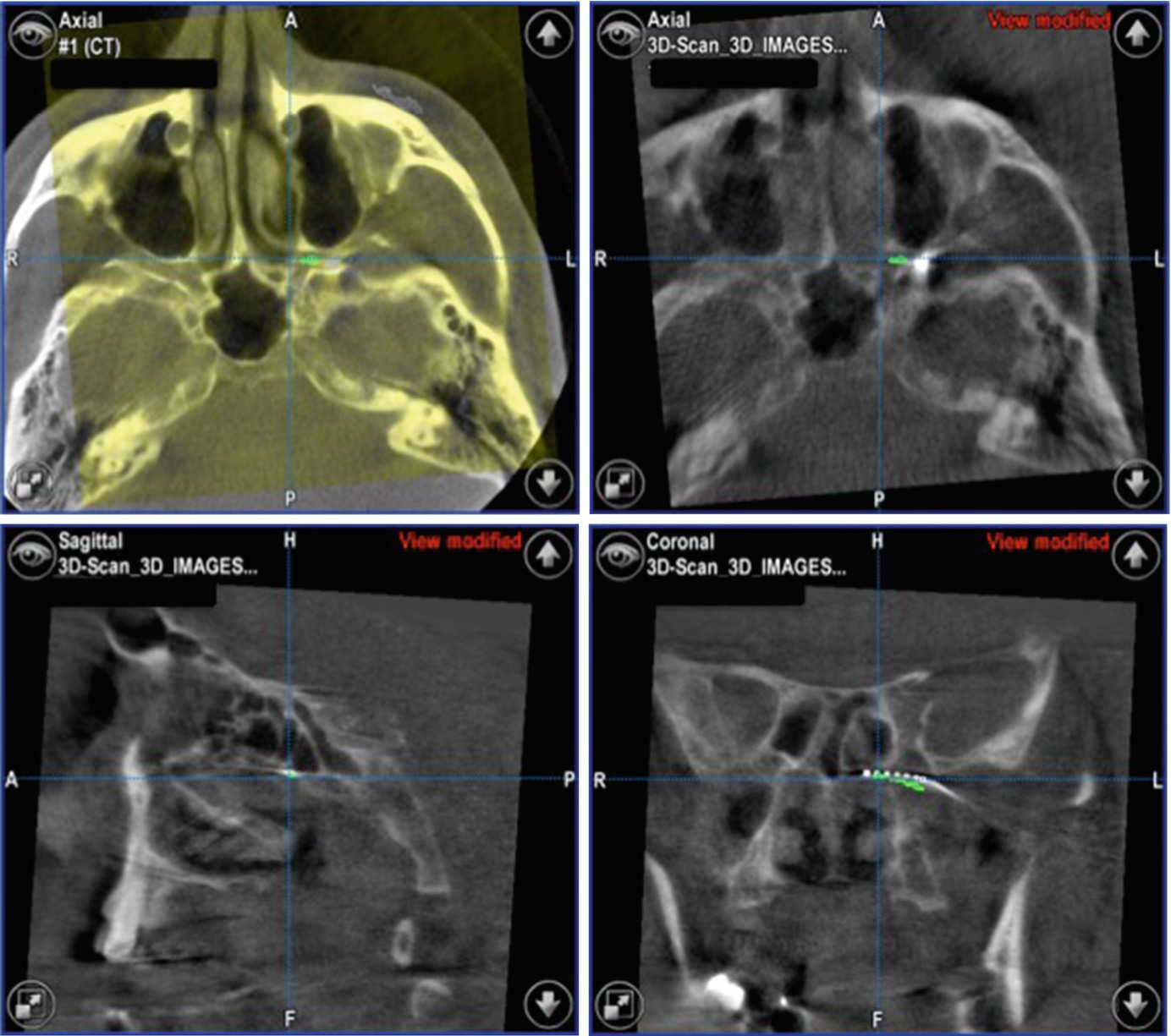
Image fusion allows the direct comparison of the expected SPG-microstimulator position and the real-time result
5.3.4 Postsurgical Aspects
It is relatively common that the patient wake up with a CH attack immediately after the general anesthesia, which the patient of course should be informed in advance. Postoperatively, patients should pay special attention to avoiding pressure on the cheek on the affected side. Diet should be limited to soft and cool food on the first postoperative day. Smoking should be avoided in the first couple of days. Oral decontamination, for example, Chlorhexidine 0.12% should be continued twice daily for 1 week. Peroral antibiotics on the first three postoperative days are recommended. One to two weeks after surgery, the wound should be inspected and sutures removed if resorbable suture is not used. Infections may be treated at this point using debridement or intravenous antibiotics. The patient can subsequently receive normal dental treatment, filling and crown therapy, etc., but the dentist should avoid injection of local anesthetic behind the tuber maxillae on the operated side to prevent damage to the electrode or inappropriate relocation of the lead.
5.3.5 Programming
First programming and stimulation can be attempted 2 weeks following insertion. Earlier attempts are generally avoided as healing is still ongoing. The adjustable parameters for the Pulsante™ system include pulse frequency, width, and amplitude. Furthermore, the anode/cathode state of the six electrodes on the lead can be changed. The overall guiding principle of programming is to obtain a sensation of parasthesia in the posterior nasopharynx. The reasoning behind this is that this is the innervation area for the sensory components of the SPG. Thus, if parasthesia is obtained here, by inference, the SPG must be in the stimulation field. The programming is done by a technician in cooperation with the patient. Several attempts, weeks apart, may be necessary before a suitable setting is found.
5.3.6 Side Effects
The microstimulator is small and, barring surgical side effects, does not give rise to cosmetic issues. However, a majority of patients experience postoperative sequalae. These include pain and swelling (47%) and sensory disruptions (67%) [14, 19, 21]. Resolution of these surgical side effects is usually swift, within 2–3 months, and would typically be classified as mild–moderate. Some patients experience a transient increase in attacks in the days and weeks following surgery. No late side effects (24 months) have been reported. Most importantly there has been no need for repeated surgery due to lead breaks or migrations [21]. Special consideration has been given to the possibility of contralateral attacks or even side shifts. However, it does not appear that that SPGS can be causally related to observed contralateral attacks, which do occur with some regularity in CH in general [22].
5.4 Clinical Evidence
5.4.1 Patient Selection
- (a)
A documented history of refractory cluster headache for at least 2 years before the implantation.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


