Pathophysiology of Headache & Facial Pain
Radiation or Projection of Pain
Classification & Approach to the Differential Diagnosis
Other Cerebrovascular Disorders
Other Causes of Acute Headache
Idiopathic Intracranial Hypertension
Persistent Idiopathic Facial Pain
Cluster Headache & Trigeminal Autonomic Cephalalgias
Headache occurs in all age groups and accounts for 1% to 2% of emergency department evaluations and up to 4% of medical office visits; the causes are myriad (Table 6-1). Although most often a benign condition (especially when chronic and recurrent), headache of new onset may be the earliest or the principal manifestation of serious systemic or intracranial disease and therefore requires thorough and systematic evaluation.
Table 6-1. Causes of Headache and Facial Pain.
Acute onset
Common causes
Subarachnoid hemorrhage
Other cerebrovascular diseases
Meningitis or encephalitis
Ophthalmic disorders (glaucoma, acute iritis)
Less common causes
Seizures
Lumbar puncture
Hypertensive encephalopathy
Coitus
Subacute onset
Giant cell (temporal) arteritis
Intracranial mass (tumor, subdural hematoma, abscess)
Pseudotumor cerebri (idiopathic intracranial hypertension)
Trigeminal neuralgia (tic douloureux)
Glossopharyngeal neuralgia
Postherpetic neuralgia
Persistent idiopathic facial pain
Chronic
Migraine
Medication overuse headache
Cluster headache
Tension-type headache
Icepick-like pain
Cervical spine disease
Sinusitis
Dental disease
An etiologic diagnosis of headache is based on understanding the pathophysiology of head pain; obtaining a history, with characterization of the pain as acute, subacute, or chronic; performing a careful physical examination; and formulating a differential diagnosis.
APPROACH TO DIAGNOSIS
PATHOPHYSIOLOGY OF HEADACHE & FACIAL PAIN
PAIN-SENSITIVE STRUCTURES
Headache is caused by traction, displacement, inflammation, or distention of the pain-sensitive structures in the head or neck. Isolated involvement of the bony skull, most of the dura, or most regions of brain parenchyma does not produce pain.
A. Pain-Sensitive Structures Within the Cranial Vault
These include the venous sinuses (eg, sagittal sinus); anterior and middle meningeal arteries; dura at the base of the skull; trigeminal (V), glossopharyngeal (IX), and vagus (X) nerves; proximal portions of the internal carotid artery and its branches near the circle of Willis; brainstem periaqueductal gray matter; and sensory nuclei of the thalamus.
B. Extracranial Pain-Sensitive Structures
These include periosteum of the skull; skin; subcutaneous tissues, muscles, and arteries; neck muscles; second (C2) and third (C3) cervical nerves; eyes, ears, teeth, sinuses, and oropharynx; and mucous membranes of the nasal cavity.
RADIATION OR PROJECTION OF PAIN
A. The trigeminal (V) nerve carries sensation from intracranial structures in the anterior and middle fossae of the skull (above the cerebellar tentorium). Discrete intracranial lesions in these locations can produce pain that radiates in the trigeminal nerve distribution (Figure 6-1).
B. The glossopharyngeal (IX) and vagus (X) nerves convey sensation from part of the posterior fossa; pain originating in this area may also be referred to the ear or throat, as in glossopharyngeal neuralgia.
C. The upper cervical (C2-C3) nerves transmit stimuli from infratentorial and cervical structures; therefore, pain from posterior fossa lesions often projects to the second and third cervical dermatomes (see Figure 6-1).
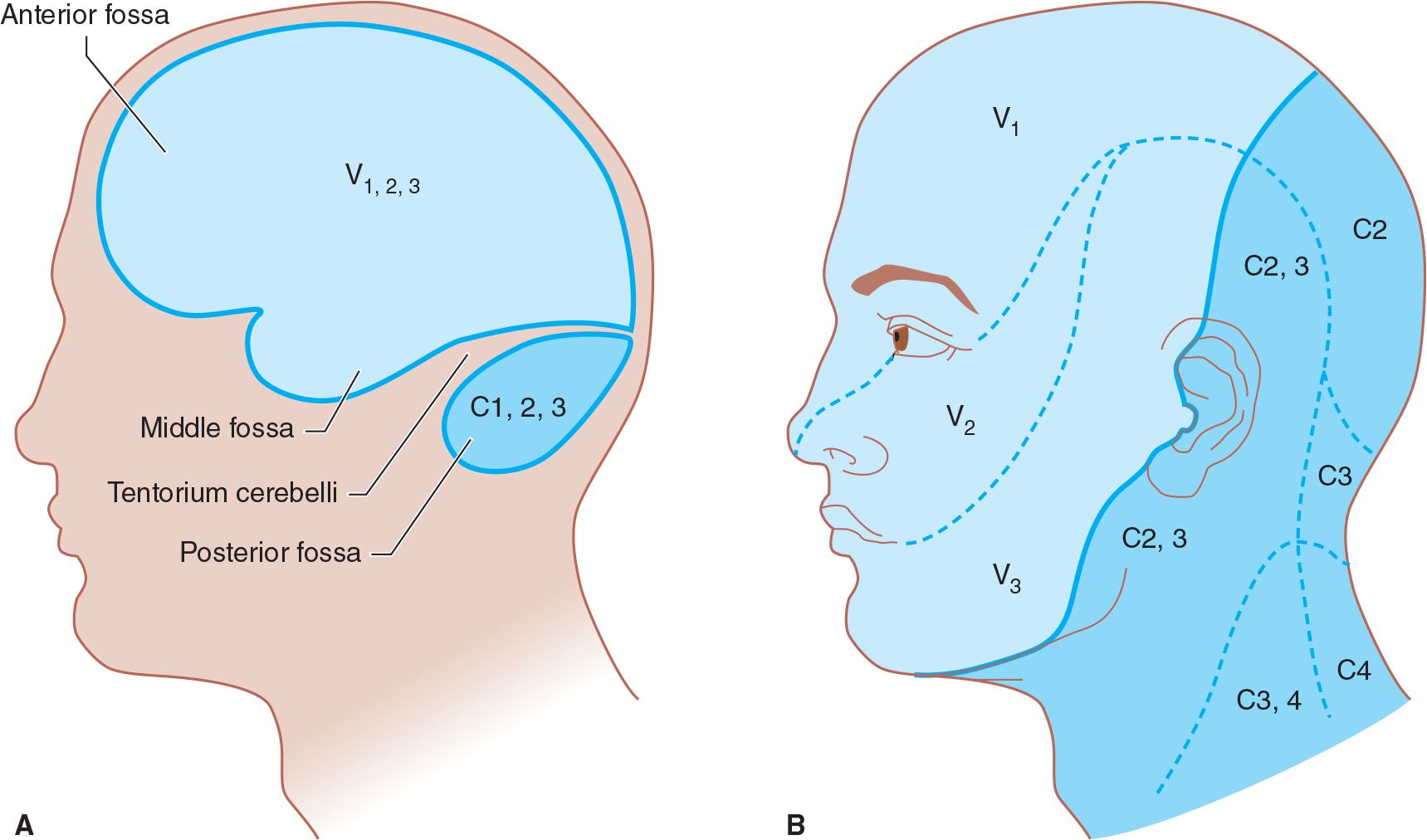
![]() Figure 6-1. Innervation of pain-sensitive intracranial compartments (A) and corresponding extracranial sites of pain radiation (B). The trigeminal (V) nerve, especially its ophthalmic (V1) division, innervates the anterior and middle cranial fossae; lesions in these areas can produce frontal headache. The upper cervical nerve roots (especially C2) innervate the posterior fossa; lesions here can cause occipital headache.
Figure 6-1. Innervation of pain-sensitive intracranial compartments (A) and corresponding extracranial sites of pain radiation (B). The trigeminal (V) nerve, especially its ophthalmic (V1) division, innervates the anterior and middle cranial fossae; lesions in these areas can produce frontal headache. The upper cervical nerve roots (especially C2) innervate the posterior fossa; lesions here can cause occipital headache.
HISTORY
CLASSIFICATION & APPROACH TO THE DIFFERENTIAL DIAGNOSIS
Acute Headache & Facial Pain
Headaches that are new in onset or clearly different from any the patient has experienced previously are commonly a symptom of serious illness and demand prompt evaluation. The sudden onset of “the worst headache I’ve ever had in my life” (classically due to subarachnoid hemorrhage), diffuse headache with neck stiffness and fever (meningitis), and head pain centered about one eye (acute glaucoma) are striking examples. Acute headaches may also accompany more benign processes such as systemic viral infections or other febrile illnesses.
Subacute Headache & Facial Pain
Subacute headaches are those that persist or recur over weeks to months. Such headaches may also signify serious medical disorders, especially when the pain is progressive or when it occurs in elderly patients. Patients with subacute headache should be asked about recent head trauma (subdural hematoma or postconcussive syndrome); malaise, fever, or neck stiffness (subacute meningitis); focal neurologic abnormalities or weight loss (primary or metastatic brain tumor); visual changes (giant cell arteritis, idiopathic intracranial hypertension); or medications predisposing to headache (nitrates).
Chronic Headache & Facial Pain
Headaches that have recurred over years (eg, migraine or tension-type headaches) usually have a benign cause, although each acute attack may be profoundly disabling. When treating these patients, it is important to determine whether the present headache is similar to those suffered previously or is new—and thus represents a different process.
PRECIPITATING FACTORS
Precipitating factors can provide a guide to the cause of headache. Such factors include: dental surgery; acute exacerbation of chronic sinusitis or hay fever; systemic viral infection; psychologic tension, emotional stress, or fatigue; menses; hunger; consumption of ice cream or foods containing nitrite (hot dogs, salami, ham, and most sausage), phenylethylamine (chocolate), or tyramine (cheddar cheese); and exposure to bright lights. Precipitation of headache by alcohol is especially typical of cluster headache. Chewing and eating commonly trigger pain associated with glossopharyngeal neuralgia and tic douloureux; jaw claudication is associated with giant cell arteritis or temporomandibular joint dysfunction. Oral contraceptive agents and nitrates may precipitate or exacerbate migraine. Headache may also be provoked by coughing or sneezing, especially in patients with structural lesions in the posterior fossa.
PRODROMAL SYMPTOMS (AURAS)
Prodromal symptoms or auras, such as scintillating scotomas or other visual changes, often occur with migraine; they may also occur in patients with seizure disorders and postictal headaches.
CHARACTERISTICS OF PAIN
Headache or facial pain may be described in a variety of ways. Pulsating or throbbing pain is frequently ascribed to migraine, but may also occur in patients with tension-type headache. A steady sensation of tightness or pressure is commonly seen with tension-type headache. The pain produced by intracranial mass lesions is typically dull and steady. Sharp, lancinating (stabbing) pain suggests a neuritic cause such as trigeminal neuralgia. Icepick-like pain may be described also by patients with migraine, cluster headache, or giant cell arteritis.
Headache of virtually any description can occur in patients with migraine or brain tumors; therefore, the character of the pain alone does not provide a reliable etiologic guide.
LOCATION OF PAIN
A. Unilateral headache is an invariable feature of cluster headache and occurs in the majority of migraine attacks; most patients with tension-type headache report bilateral pain.
B. Ocular or retroorbital pain suggests a primary ophthalmic disorder such as acute iritis or glaucoma, optic (II) nerve disease (eg, optic neuritis), or retroorbital inflammation (eg, Tolosa–Hunt syndrome). It is also common in migraine or cluster headache.
C. Paranasal pain localized to one or several sinuses, often associated with tenderness of the overlying periosteum and skin, occurs with acute sinus infection or outlet obstruction.
D. Focal headache may result from intracranial mass lesions, but even in such cases it is replaced by bioccipital and bifrontal pain when the intracranial pressure becomes elevated.
E. Bandlike or occipital discomfort is commonly associated with tension-type headache. Occipital localization can also occur with meningeal irritation from infection or hemorrhage and with disorders of the joints, muscles, or ligaments of the upper cervical spine.
F. Pain within the first (V1) division of the trigeminal nerve, characteristically described as burning in quality, is a common feature of postherpetic neuralgia.
G. Lancinating pain localized to the second (V2) or third (V3) division of the trigeminal (V) nerve suggests trigeminal neuralgia (tic douloureux).
H. The pharynx and external auditory meatus are the most frequent sites of pain caused by glossopharyngeal neuralgia.
ASSOCIATED SYMPTOMS
Manifestations of underlying systemic disease can aid in the etiologic diagnosis of headache and should always be sought.
A. Recent weight loss may accompany cancer, giant cell arteritis, or depression.
B. Fever or chills may indicate systemic infection or meningitis.
C. Dyspnea or other symptoms of heart disease raise the possibility of subacute infective endocarditis and resultant brain abscess.
D. Visual disturbances suggest an ocular disorder (eg, glaucoma), migraine, or an intracranial process involving the optic nerve (optic neuritis) or tract or central visual pathways.
E. Nausea and vomiting are common in migraine and post-traumatic headache and can also be seen with intracranial mass lesions. Some patients with migraine report that diarrhea or other gastrointestinal symptoms accompany attacks.
F. Photophobia may be prominent in migraine, acute meningitis, or subarachnoid hemorrhage.
G. Myalgias often accompany tension-type headache, systemic viral infections, and giant cell arteritis.
H. Ipsilateral rhinorrhea and lacrimation during attacks typify cluster headache.
I. Transient loss of consciousness may be seen in both migraine (basilar migraine) and glossopharyngeal neuralgia (due to cardiac syncope; Chapter 12, Seizures & Syncope).
OTHER FEATURES OF HEADACHE
Temporal Pattern of Headache
Headaches from mass lesions are commonly maximal on awakening, as are sinus headaches. Headaches from mass lesions, however, increase in severity over time. Cluster headaches frequently awaken patients from sleep; they often recur at the same time each day or night. Tension-type headaches can develop whenever stressful situations occur and are often maximal at the end of a workday. Migraine headaches are episodic and may be worse during menses (Figure 6-2).
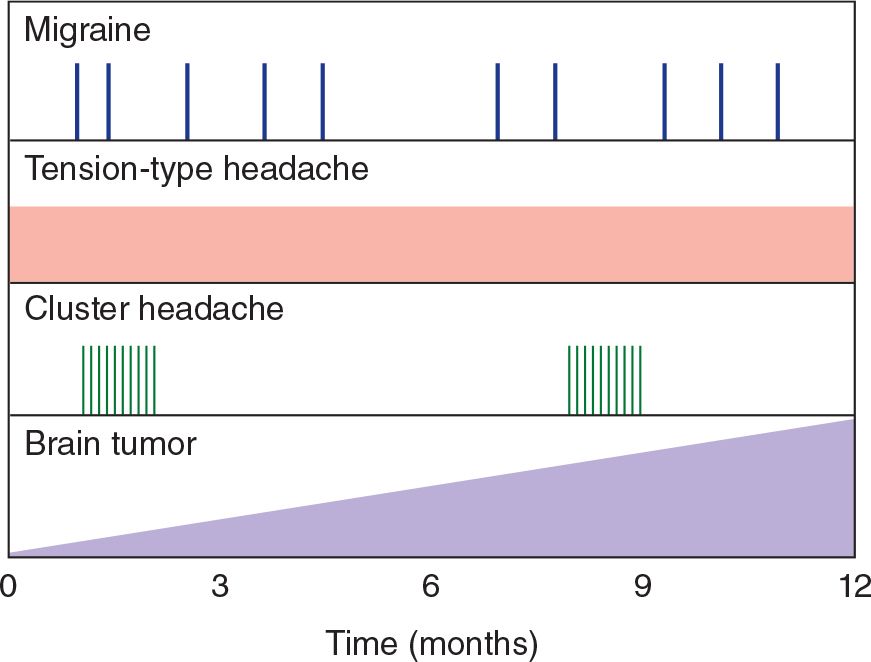
![]() Figure 6-2. Temporal patterns of headache. Migraine headache is episodic and may occur at varying intervals. Tension-type headache may be present every day. Cluster headache occurs in bouts separated by symptom-free periods. Headache caused by brain tumor often increases in severity with time.
Figure 6-2. Temporal patterns of headache. Migraine headache is episodic and may occur at varying intervals. Tension-type headache may be present every day. Cluster headache occurs in bouts separated by symptom-free periods. Headache caused by brain tumor often increases in severity with time.
Conditions Relieving Headache
Migraine headaches are frequently relieved by darkness, sleep, vomiting, or pressing on the ipsilateral temporal artery, and their frequency may diminish during pregnancy. Post–lumbar-puncture and low-pressure headaches are typically relieved by recumbency, whereas headaches caused by intracranial mass lesions may be less severe with the patient standing.
Conditions Exacerbating Headache
Discomfort exacerbated by rapid changes in head position or by events that transiently raise intracranial pressure, such as coughing and sneezing, is often associated with an intracranial mass but can also occur in migraine. Anger, excitement, or irritation can precipitate or worsen both migraine and tension-type headaches. Stooping, bending forward, sneezing, or blowing the nose characteristically worsens the pain of sinusitis. Postural headache (maximal when upright, nearly absent when lying down) occurs with low cerebrospinal fluid (CSF) pressure caused by lumbar puncture, head injury, or spontaneous spinal fluid leak. Fluctuations in intensity and duration of the headache with no obvious cause, especially when associated with similar fluctuations in mental status, are seen with subdural hematoma.
History of Headache
The characteristics of the present headache should be compared with those of previous occurrences, because headache with features different from those previously experienced calls for especially careful investigation.
PHYSICAL EXAMINATION
A general physical examination is mandatory, because headache is a nonspecific accompaniment of many systemic disorders. If possible, the patient should be observed during an episode of headache or facial pain.
VITAL SIGNS
Temperature
Although fever suggests a viral syndrome, meningitis, encephalitis, or brain abscess, headache from these causes can also occur without fever. Moreover, headache can accompany many systemic infections.
Pulse
Tachycardia can occur in a tense, anxious patient with a tension-type headache or accompany any severe pain. Paroxysmal headache associated with tachycardia and perspiration is characteristic of pheochromocytoma.
Blood Pressure
Hypertension per se rarely causes headache unless the blood pressure elevation is acute, as with pheochromocytoma, or very high, as with early hypertensive encephalopathy. Chronic hypertension, however, is the major risk factor for hemorrhagic or ischemic stroke, which can be associated with acute headache. Subarachnoid hemorrhage is commonly followed by marked acute elevation of blood pressure.
Respiration
Hypercapnia from respiratory insufficiency from any cause can elevate intracranial pressure and produce headache.
GENERAL PHYSICAL EXAMINATION
Weight Loss
Weight loss or cachexia in a patient with headache suggests cancer or chronic infection. Polymyalgia rheumatica and giant cell arteritis can also be accompanied by weight loss.
Skin
Focal inflammation (cellulitis) of the face or overlying the skull indicates local infection, which may be the source of intracranial abscess or cause venous sinus thrombosis. Cutaneous abnormalities elsewhere may suggest vasculitis (including that from meningococcemia), endocarditis, or cancer. The neurofibromas or café-au-lait spots of von Recklinghausen disease (neurofibromatosis) may be associated with benign or malignant intracranial tumors that can produce headache. Cutaneous angiomas sometimes accompany arteriovenous malformations (AVMs) of the central nervous system, which may be associated with chronic headache—or acute headache if they bleed. Herpes zoster that affects the face and head most often involves the eye and the skin around the periorbital tissue, causing facial pain.
Scalp, Face, & Head
Scalp tenderness is characteristic of migraine headache, subdural hematoma, giant cell arteritis, and postherpetic neuralgia. Nodularity, erythema, or tenderness over the temporal artery suggests giant cell arteritis. Localized tenderness of the superficial temporal artery also accompanies acute migraine. Recent head trauma or a mass lesion can cause a localized area of tenderness. Trauma causes characteristic ecchymosis (see Figure 1-4).
Paget disease, myeloma, or metastatic cancer of the skull may produce head pain that is boring in quality and associated with skull tenderness. In Paget disease, arteriovenous shunting within bone may make the scalp feel warm.
Disorders of the eyes, ears, or teeth may cause headache. Tooth percussion may reveal periodontal abscess. Sinus tenderness may indicate sinusitis. A bruit over the orbit or skull suggests an intracranial AVM, carotid artery–cavernous sinus fistula, aneurysm, or meningioma. Lacerations of the tongue raise the possibility of postictal headache. Ipsilateral conjunctival injection, lacrimation, Horner syndrome (see Chapter 7, Neuro-Ophthalmic Disorders), and rhinorrhea occur with cluster headache. Temporomandibular joint disease is accompanied by local tenderness and crepitus over the joint.
Neck
Cervical muscle spasm occurs with tension-type and migraine headaches, cervical spine injuries, cervical arthritis, or meningitis. Carotid bruits may be associated with cerebrovascular disease.
Meningeal signs must be sought carefully, especially if headache is of recent onset. Meningeal irritation causes nuchal (neck) rigidity mainly in the anteroposterior direction, whereas cervical spine disorders restrict movement in all directions. Discomfort or hip and knee flexion during neck flexion (Brudzinski sign) indicates meningeal irritation (see Figure 1-5). Meningeal signs may be absent or difficult to demonstrate in the early stages of subacute (eg, tuberculous) meningitis, in the first few hours after subarachnoid hemorrhage, and in comatose patients.
Heart & Lungs
Brain abscess may be associated with congenital heart disease, which is evidenced by heart murmur or cyanosis. Lung abscess may also be a source of brain abscess.
NEUROLOGIC EXAMINATION
Mental Status Examination
During the mental status examination, patients with acute headache may be confused, as is commonly seen with subarachnoid hemorrhage and meningitis. Headache with dementia may be indicative of intracranial tumor, particularly one in the frontal lobe or infiltrating across the corpus callosum.
Cranial Nerve Examination
Cranial nerve abnormalities can suggest and localize an intracranial tumor or other mass lesion. Papilledema, the hallmark of increased intracranial pressure, may be seen with space-occupying intracranial lesions, carotid artery–cavernous sinus fistula, pseudotumor cerebri, or hypertensive encephalopathy. Superficial retinal hemorrhages (subhyaloid hemorrhages) are characteristic of subarachnoid hemorrhage in adults (Figure 6-3). Ischemic retinopathy may be found in patients with vasculitis.
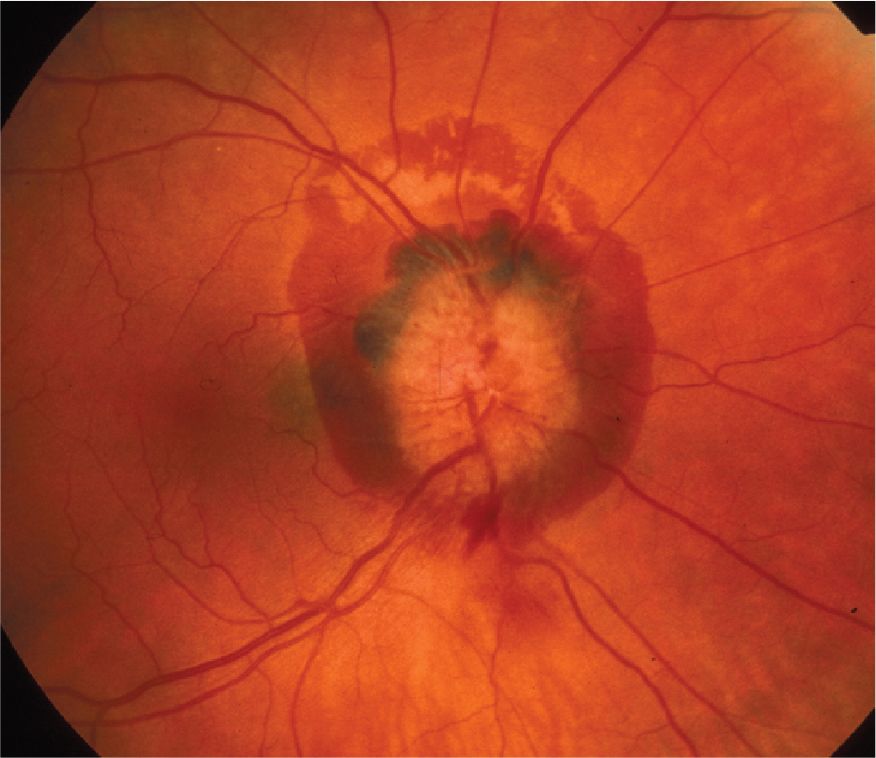
![]() Figure 6-3. Disc edema and a large peripapillary subhyaloid hemorrhage in the right eye of a patient with aneurysmal rupture and subarachnoid hemorrhage. (Used with permission from Biousse V, Newman NJ. Neuro-Ophthalmology Illustrated. Thieme, NY. 2009.)
Figure 6-3. Disc edema and a large peripapillary subhyaloid hemorrhage in the right eye of a patient with aneurysmal rupture and subarachnoid hemorrhage. (Used with permission from Biousse V, Newman NJ. Neuro-Ophthalmology Illustrated. Thieme, NY. 2009.)
Progressive oculomotor (III) nerve palsy, especially when it causes pupillary dilatation, may be the presenting sign of an expanding posterior communicating artery aneurysm; alternatively, it may reflect increasing intracranial pressure and incipient brain herniation. Decreased pupillary reactivity to light occurs in optic neuritis. Extraocular muscle palsies occur in Tolosa-Hunt syndrome (see Chapter 7, Neuro-Ophthalmic Disorders). Proptosis suggests an orbital mass lesion or carotid artery–cavernous sinus fistula.
Decreased sensation over the site of facial pain—most commonly the first (V1) division of the trigeminal (V) nerve—is found in postherpetic neuralgia. Trigger areas on the face and pharynx suggest trigeminal and glossopharyngeal neuralgia, respectively.
Motor Examination
Asymmetric motor function or gait ataxia in a patient with a history of subacute headache demands complete evaluation to exclude intracranial mass lesions.
Sensory Examination
Focal or segmental sensory impairment or diminished corneal sensation (corneal reflex) is strong evidence against a benign cause of pain.
ACUTE HEADACHE
Sudden onset of a new headache may be a symptom of serious intracranial or systemic disease; it must be investigated promptly and thoroughly.
SUBARACHNOID HEMORRHAGE
Spontaneous (nontraumatic) subarachnoid hemorrhage (bleeding into the subarachnoid space) is usually the result of a ruptured cerebral arterial aneurysm or an AVM.
Rupture of a saccular (berry) aneurysm accounts for approximately 75% of cases of subarachnoid hemorrhage, with an annual incidence of 6 per 100,000. Most arise sporadically, but some are familial. Families with two or more affected persons should have all members screened. Both autosomal and recessive patterns of inheritance occur.
Rupture occurs most often during the fifth and sixth decades, with an approximately equal sex distribution. The risk of rupture of an intracranial aneurysm varies with the patient’s age and the site and size of the aneurysm; approximately 5% of autopsied individuals have cerebral aneurysms, and most have never experienced symptoms. Hypertension has not been conclusively demonstrated to predispose to the formation of aneurysms, but acute elevation of blood pressure (eg, at orgasm) may be responsible for rupture.
Fusiform aneurysms result from circumferential dilation of a cerebral arterial trunk. In contrast to saccular aneurysms, they are thought to be caused by atherosclerosis or dissection, affect the vertebrobasilar system preferentially, and can present with symptoms from ischemia or mass effect, in addition to rupture.
Intracranial AVMs, a less frequent cause of subarachnoid hemorrhage (10%), occur twice as often in men as in women, and usually bleed in the second to fourth decades, although a significant incidence extends into the sixties. Blood in the subarachnoid space can also result from intracerebral hemorrhage, embolic stroke, and trauma.
PATHOLOGY
Cerebral arterial aneurysms are usually congenital and result from developmental weakness of the vessel wall, especially at sites of branching. They typically arise from intracranial arteries about the circle of Willis at the base of the brain (Figure 6-4), occur in 2% of patients, and are multiple in approximately 20% of cases. Other congenital abnormalities, such as polycystic kidney disease or coarctation of the aorta, may be associated with berry aneurysms.
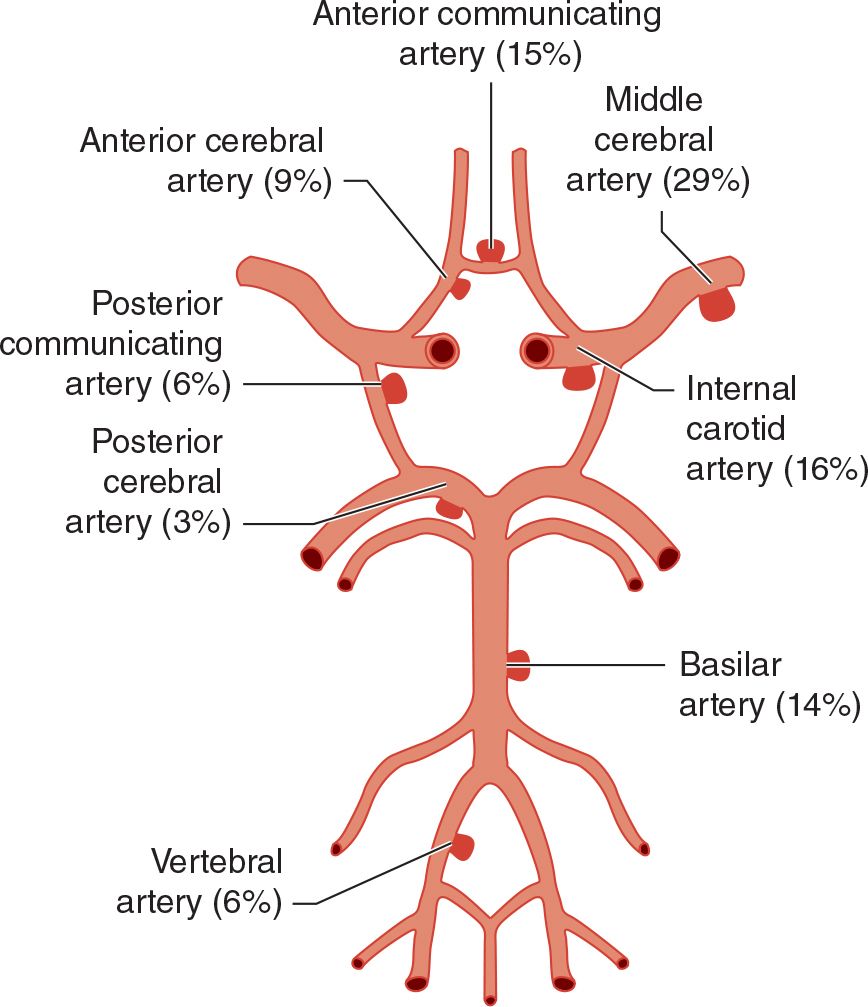
![]() Figure 6-4. Frequency distribution of intracranial aneurysms.
Figure 6-4. Frequency distribution of intracranial aneurysms.
Occasionally, systemic infections such as infective endocarditis disseminate to a cerebral artery and cause aneurysm formation; such mycotic aneurysms account for 2% to 3% of aneurysmal ruptures. Mycotic aneurysms are usually more distal along the course of cerebral arteries than are berry aneurysms.
AVMs consist of abnormal vascular communications that permit arterial blood to enter the venous system without passing through a capillary bed. They are most common in the middle cerebral artery distribution.
PATHOPHYSIOLOGY
Rupture of an intracranial artery elevates intracranial pressure and distorts pain-sensitive structures, producing headache. Intracranial pressure may reach systemic perfusion pressure and acutely decrease cerebral blood flow; together with the concussive effect of the rupture, this is thought to cause the loss of consciousness that occurs at the onset in approximately 50% of patients. Rapid elevation of intracranial pressure can also produce subhyaloid retinal hemorrhages (see Figure 6-3).
CLINICAL FINDINGS
Symptoms & Signs
The classic (but not invariable) presentation of subarachnoid hemorrhage is with the sudden onset of an unusually severe generalized headache, classically described as the worst headache the patient has ever experienced. However, among patients presenting with the abrupt onset of an unusually severe headache, only 8% to 10% will have subarachnoid hemorrhage. The absence of headache essentially precludes the diagnosis. One-third of patients present with headache alone. In the remainder, loss of consciousness is frequent at onset, as are vomiting and neck stiffness. Symptoms may begin at any time of day and during either rest or exertion.
The most significant feature of the headache is that it is new. Milder but otherwise similar headache (sentinel headache) may have occurred in the weeks prior to the acute event and probably represent small prodromal hemorrhages or aneurysmal stretch.
The headache is not always severe, especially if hemorrhage is from a ruptured AVM rather than an aneurysm. Although the duration of the hemorrhage is brief, the intensity of the headache may remain unchanged for several days and may subside slowly over approximately 2 weeks. Recurrence of the headache usually signifies rebleeding.
Blood pressure frequently rises precipitously as a result of the hemorrhage. Meningeal irritation may induce temperature elevations up to 39°C (102.2°F) during the first 2 weeks. There is frequently associated confusion, stupor, or coma. Nuchal rigidity and other evidence of meningeal irritation (see Figure 1-5) are common, but may not occur for several hours after the onset of headache. Preretinal globular subhyaloid hemorrhages (found in 20%-40% of cases; see Figure 6-3) are most suggestive of the diagnosis.
With aneurysmal rupture, bleeding occurs mainly in the subarachnoid space rather than brain parenchyma. Therefore, prominent focal neurologic signs are uncommon, and even when present, they may bear no relationship to the site of the aneurysm. An exception is oculomotor (III) nerve palsy from compression of the nerve ipsilateral to a posterior communicating artery aneurysm. Bilateral extensor plantar responses and abducens (VI) nerve palsy are frequent nonlocalizing signs that result from increased intracranial pressure.
Ruptured AVMs tend to occur within brain tissue and accordingly produce focal neurologic signs, such as hemiparesis, aphasia, or visual field defects.
Laboratory Findings
Patients presenting with subarachnoid hemorrhage are investigated first by computed tomography (CT) scan (Figure 6-5), which confirms the hemorrhage in more than 90% of patients with aneurysmal rupture and can help identify a focal source. The test is most sensitive on the day bleeding occurs and in patients with altered consciousness. Complications, including intracerebral or intraventricular extension of blood, hydrocephalus, and infarction, can also be identified. Aneurysms themselves may not be evident on CT, but most AVMs can be seen after administration of contrast material. Magnetic resonance imaging (MRI) is especially useful for detecting small AVMs in the brainstem, which are poorly seen on CT scan.
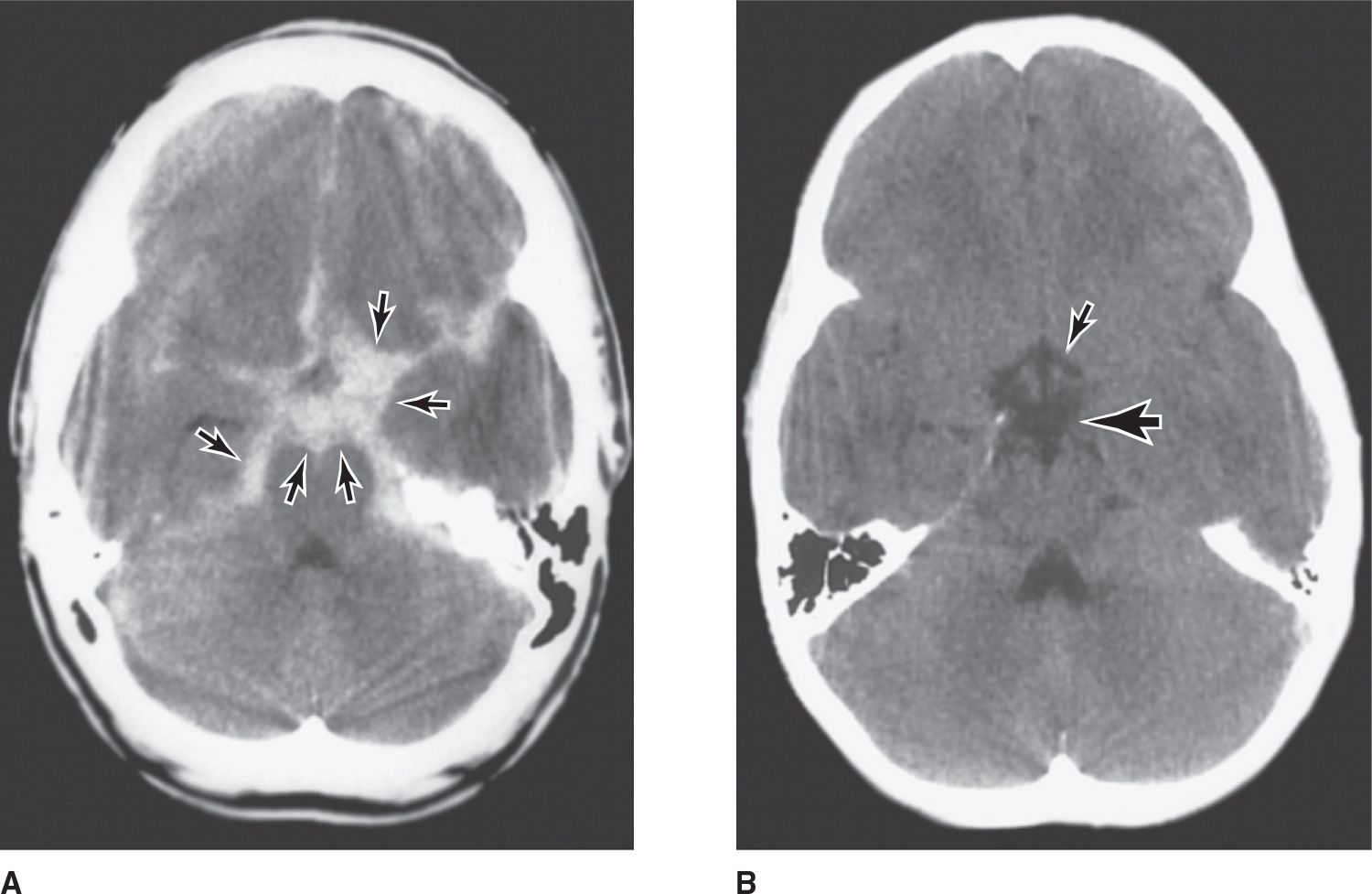
![]() Figure 6-5. (A) Nonenhanced brain CT scan from a patient with an acute aneurysmal subarachnoid hemorrhage. Areas of high density (arrows) represent blood in the subarachnoid space at the base of the brain (most aneurysms occur in this region about the circle of Willis; see Figure 6-4). (B) A normal nonenhanced brain CT scan of the same region. Interpeduncular cistern, large arrow; suprasellar cistern, small arrow. (Used with permission from C. Jungreis.)
Figure 6-5. (A) Nonenhanced brain CT scan from a patient with an acute aneurysmal subarachnoid hemorrhage. Areas of high density (arrows) represent blood in the subarachnoid space at the base of the brain (most aneurysms occur in this region about the circle of Willis; see Figure 6-4). (B) A normal nonenhanced brain CT scan of the same region. Interpeduncular cistern, large arrow; suprasellar cistern, small arrow. (Used with permission from C. Jungreis.)
In patients with a normal neurologic examination, a normal CT scan within 6 hours of symptom onset is held by some authorities to exclude subarachnoid hemorrhage. CT scans that are technically inadequate or delayed, or that otherwise fail to confirm the diagnosis of subarachnoid hemorrhage, necessitate lumbar puncture (see Chapter 2, Investigative Studies). The CSF in subarachnoid hemorrhage usually has a markedly elevated opening pressure and is grossly bloody, containing 100,000 to >1 million/μL red blood cells. As heme from these cells is degraded, first (by heme oxygenase) to the green pigment, biliverdin, and then (by biliverdin reductase) to the yellow pigment, bilirubin, the supernatant of the centrifuged CSF becomes yellow-tinged (xanthochromic) within 12 hours after hemorrhage. The pigment can be detected spectrophotometrically. White blood cells are initially present in the CSF in the same proportion to red cells as in the peripheral blood. However, chemical meningitis caused by blood in the subarachnoid space may produce a pleocytosis of several thousand white blood cells during the first 48 hours and a reduction in CSF glucose 4 to 8 days after hemorrhage. In the absence of pleocytosis, CSF glucose after subarachnoid hemorrhage is normal. The peripheral white blood cell count is often modestly elevated but rarely exceeds 15,000/μL.
The electrocardiogram (ECG) may reveal a host of abnormalities, including peaked or deeply inverted T waves, short PR interval, or tall U waves.
Once the diagnosis of subarachnoid hemorrhage is confirmed by CT or lumbar puncture, four-vessel cerebral arteriography is undertaken. Both the carotid and vertebral arteries should be studied to visualize the entire cerebral vascular anatomy, as multiple aneurysms occur in 20% of patients and AVMs are frequently supplied from multiple vessels. Angiography should be performed at the earliest time convenient for radiology department personnel; emergency studies in the middle of the night are rarely indicated. Angiography is a prerequisite to the rational planning of surgical treatment and is therefore not necessary for patients who are not surgical candidates, such as those who are deeply comatose. CT and magnetic resonance angiography are approaching the sensitivity of conventional catheter angiography.



