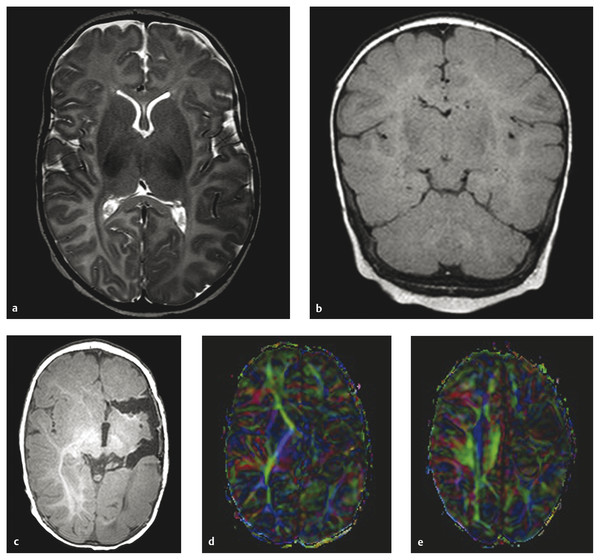
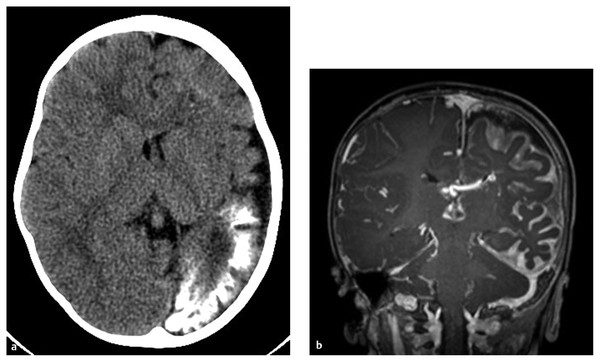
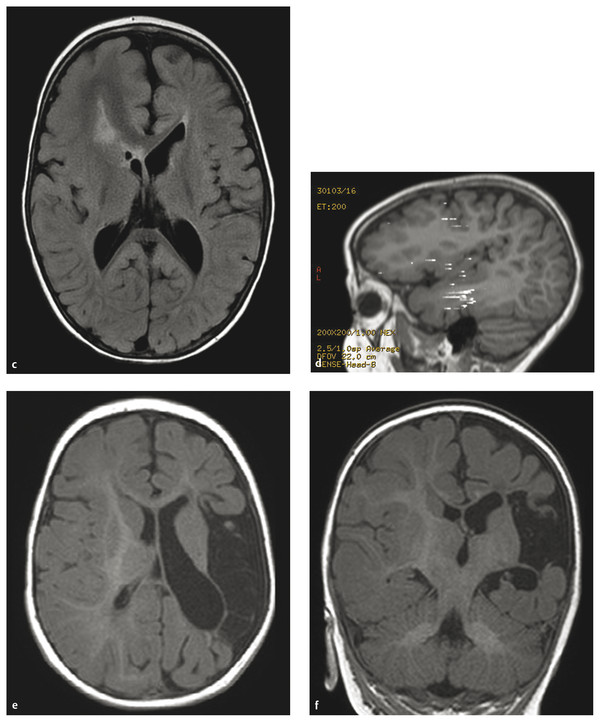
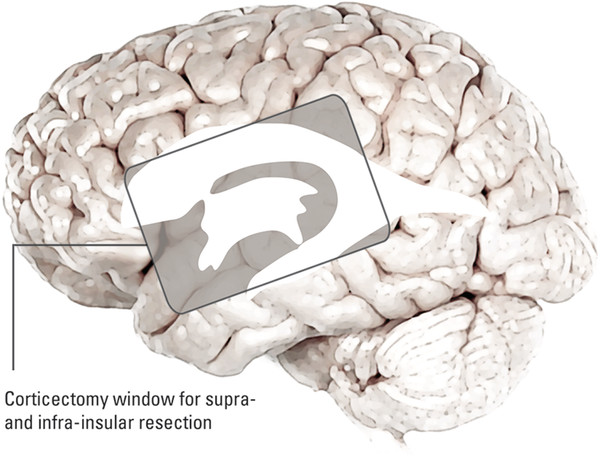
Hemispherectomy Intractable epilepsy is a devastating disease, not only for the patient and family but also for the primary healthcare provider, neurologist, and treating neurosurgeon. Neurosurgical options include invasive monitoring with tailored resection, multilobar removal, multiple subpial transections, and hemispherectomy involving a variety of techniques. This chapter provides an overview of hemispherectomy—its history, the neurosurgical options and indications, techniques, and outcome. The removal of one cerebral hemisphere, or hemispherectomy, was initially described for the control and potential cure of malignant glioma. In 1928, Dandy described a series of five patients in whom an anatomical hemispherectomy was performed by ligating the main cerebral arteries, dissecting the hemisphere off the falx medially, and incising along the corpus callosum and internal capsule, thus delivering the hemisphere.1 Gardner also published his results on hemispherectomy for gliomas in 1933.2 Although the mortality of the procedure was high, on the order of 40 to 66%, both surgeons felt that the risk could be countered by an extended life span.1,2 Sadly, improved survival was never fully realized, and patients lived out the remainder of their lives with the added functional impairments created by the surgery. McKenzie was the first to perform a hemispherectomy for epilepsy in 1938. The patient was a woman who had intractable epilepsy associated with infantile hemiplegia. Krynauw presented a series of 12 patients with infantile hemiplegia and popularized the technique in 1950.3–5 Krynauw reported that the main indication for surgery was not preoperative hemiplegia; rather, it was persistence of seizures and resultant cognitive impairment. In his experience, focal surgery might occasionally stop the seizures, but there would be no improvement in mental functioning or physical disability. Krynauw believed that any residual diseased hemisphere would continue to emit electrical discharges that would eventually spread to and involve the anatomically normal hemisphere, thereby potentially continuing to affect intellect as well as inducing secondary foci of epileptogenesis. Thus, he advocated removal of the entire hemisphere.5 The initial enthusiasm for hemispherectomy waned when in 1966 Oppenheimer and Griffith reported 3 delayed deaths in a series of 17 patients with anatomical hemispherectomy. Autopsies revealed obstructive hydrocephalus with superficial hemosiderosis, which was thought to be due to repeated episodes of bleeding within the cranial cavity. They suggested that to decrease the risk for hemosiderosis, the abnormal hemisphere should be functionally disconnected and left in place with an intact ventricular lining or that, as an alternative, the ipsilateral ventricular cavity should be occluded from contact with the other side.6 Modifications and variations of the neurosurgical technique ensued. Wilson initially discussed decreasing the volume of the hemispheric cavity in 1968, and Adams further illustrated this concept in 1983 with his “Oxford” modification, which consisted of two steps: First, the ipsilateral foramen of Monro was plugged with muscle to prevent the drainage of blood products into the ventricles; second, the dura was stripped off the bone and tacked to the falx, the tentorium, and the floor of anterior and middle cranial fossae to reduce the volume of the cavity and thus expand the extradural space.7–9 The Peacock modification included drainage of the hemispherectomy cavity with a subdural peritoneal shunt.10 Anatomical hemispherectomy achieves good seizure control, with 80 to 90% of patients obtaining an Engel Class I or II outcome.11,12 In 1974, Rasmussen developed the functional hemispherectomy. This was after subtotal hemispherectomies had been performed in a series of 48 patients from 1937 to 1971 to decrease the risk for hemosiderosis yet resulted in worsened seizure control.9 Rasmussen’s procedure involved a subtotal anatomical hemispherectomy with disconnection of the remaining cerebrum from the contralateral hemisphere to obtain the same overall result as that of an anatomical hemispherectomy.9,13 The remaining tissue protected the brain from hemosiderosis, but the result was similar to that of an anatomical hemispherectomy, with a seizure-free rate of 75%.3 As well, the incidence of postoperative hydrocephalus was less because a greater amount of the subarachnoid space was left intact.13 Other modifications to Rasmussen’s first description of functional hemispherotomy have been made. The techniques differ from one another in regard to the volume of resected brain; the route of access to the lateral ventricle; whether insular resection, hippocampal resection, or disconnection is performed; and whether there is vascular preservation or sacrifice in the peri-insular area.3 The term hemispherotomy describes a small amount of cerebral resection with extensive disconnection. In the 1990s, Delalande and colleagues described a vertical approach, while Villemure et al and Schramm et al described a lateral approach.3,8,14,15 Seizure freedom rates ranged from 74 to 85%.3,16 Details are further elucidated in the section on surgical technique. In the initial description, hemidecortication or hemicortication was similar to hemispherectomy; however, instead of removal of the hemisphere en bloc, a small amount of white matter was left over the ventricular system.17 Winston in 1992 and Hoffman in 1993 further detailed the procedure as “degloving” of the cerebrum because the cortical gray matter was resected down to the white matter, with preservation of the underlying white matter and ventricular system.3,12,18 This prevented bloody contamination of the ventricular system. The main problem with this procedure is blood loss and the technical difficulty of performing complete cortical resections on the medial and basal sides of the lobes.3,19,20 However, hemidecortication has resulted in long-term seizure freedom in 50 to 80% of patients, depending on their underlying disorder. If the anatomy of a hemispherectomy is unfavorable to ventricular approaches, then hemidecortication should be considered for patients with severe seizure disorders.20 To identify a surgical candidate, the concept of medically intractable seizures is raised. Interestingly, what defines medically intractable seizures is not always agreed upon in the literature. Intractability is defined as failure of three first-line antiepileptic medications in infants, whereas in adults, a failure of two antiepileptic medications over 2 years has been used to define intractability. However, in infants with frequent seizures, the duration of drug trials may be brief, and there is a risk with waiting because irreversible cognitive deficits may develop. As well, an early age at seizure onset, a remote symptomatic etiology, infantile spasms, status epilepticus, a poor response to short-term antiepileptic therapy, and failure of an initial antiepileptic drug trial can all predict intractability.21 Other indications for surgery include a seizure disorder limited to one hemisphere for which multifocal resections would seem inadequate.22 Preoperative hemiparesis or hemiplegia is preferable because it lessens the postoperative deficit; however, it is not mandatory because there are certain progressive conditions with a well-known natural history that result in the worsening of motor and cognitive function. Catastrophic infantile epilepsy syndromes causing multiple daily seizures put the brain at developmental risk. Therefore, these children may be considered candidates for early surgery because hemispherectomy may avert a decline in cognitive function.3 Diffuse hemispheric disorders for which hemispherectomy has been employed include Sturge-Weber syndrome, cortical dysplasia, hemimegalencephaly, Rasmussen syndrome, porencephalic cyst, and hemiconvulsion–hemiplegia–epilepsy syndrome.3,22 (▶ Fig. 75.1 and ▶ Fig. 75.2) Fig. 75.1 Hemimegalencephaly. (a) Axial T2-weighted image showing thickening in the left occipital, temporal, and parietal lobes. (b) Coronal FLAIR (fluid-attenuated inversion recovery) image revealing enlargement of the left occipital and temporal lobes. (c) T1-weighted image 6 months after hemispherotomy. (d,e) Diffusion tensor imaging elucidating the functional disconnection of the left hemisphere. Fig. 75.2 Illustrative imaging studies depicting the neuropathology of cases for which hemispherotomy is indicated. (a,b) Axial computed tomographic scan and coronal T1-weighted magnetic resonance (MR) image with contrast of Sturge-Weber syndrome. (c,d) Axial FLAIR (fluid-attenuated inversion recovery) MR image and magnetoelectroencephalogram of hemimegalencephaly. (e,f) Axial and coronal T1-weighted MR images of perinatal middle cerebral artery infarct. Patients with Sturge-Weber syndrome typically have hypertrophic pial vessels, and the venous sinuses and veins are frequently absent. The result is a strong retrograde venous flow into the ventricles, and the aberrant cerebral vasculature creates a hypoxic environment as blood is shunted away from the parenchyma. This leads to cellular damage and secondary seizures. Patients typically present with seizures in the first year of life, which begin as simple or complex partial seizures that can secondarily generalize. In patients with diffuse Sturge-Weber syndrome, hemispherectomy is recommended early because their condition will progress to intractable epilepsy, hemiplegia, and cognitive impairment.3,22 Intractable epilepsy is seen in patients with unilateral multilobar or extensive cortical dysplasia. Cortical dysplasia has been described as the most common underlying surgical pathology in children with intractable epilepsy. It is characterized by a disturbance of the normal cortical lamination, with resulting abnormal cells and neuronal circuitry that lead to increased epileptogenicity. The epilepsy type depends on the amount and location of cortical dysplasia but is usually chronic and consists of partial or generalized seizures.3,23 Hemimegalencephaly is a hemispheric neuronal migrational disorder. An enlarged hemisphere with lamination of only three to four layers, heterotopias, flat gyri, giant neurons, shallow sulci, poor gray–white matter differentiation, and an abnormal ventricular system characterize this disorder. Partial seizures with generalization lead to intractable epilepsy. Epileptic encephalopathy and developmental delay are the outcomes for these patients if they are not aggressively treated.3,22 Rasmussen syndrome is an acquired progressive disease described as chronic encephalitis with spreading cortical inflammation that results in seizures, typically epilepsia partialis continua. The patients experience intellectual deterioration and progressive hemiparesis.3,22 Porencephalic cysts result from perinatal insults and traumatic brain injuries. Etiologies include large infarcts, intracranial hemorrhage, and coagulopathies. The epilepsy is typically medically intractable, and hemiplegia is commonly found.3 Hemiconvulsion–hemiplegia–epilepsy syndrome has three phases. It initially presents with unilateral, prolonged clonic seizures that affect the face, arm, and leg. This is followed by hemiplegia and partial epileptic seizures. Chronic epilepsy evolves within 1 to 2 years. The etiology of this disease is unknown.3 At The Hospital for Sick Children, all patients are evaluated by an experienced pediatric epileptologist, an epilepsy surgeon, and a neuropsychologist if age permits, and they are discussed at a multidisciplinary epilepsy conference. Ancillary studies include interictal and ictal scalp video electroencephalography (EEG), magnetic resonance (MR) imaging, and frequently positron emission tomography (PET) and functional MR imaging (fMRI). Wada testing is not always feasible in children and also carries a risk for ischemia of the unaffected hemisphere.3 Patients at The Hospital for Sick Children routinely undergo magnetoencephalography (MEG). Torres et al recently reported on the contribution of MEG to patient selection for hemispherectomy. With a small cohort, they found that MEG correlated with video EEG in most patients; however, a lack of MEG spikes in the contralateral hemisphere was not a predictive factor for seizure-free outcome.24 A larger cohort will likely allow further delineation of the information obtained from MEG.3 EEG will help determine if the epileptiform discharges are limited to one hemisphere. As well, predictors of good outcome are the following: ipsilateral suppression of electrical activity associated with multifocal epileptiform abnormalities confined to the damaged hemisphere; bilateral synchronous discharges spreading from the abnormal hemisphere without contralateral slowing; and the absence of generalized discharges, bilateral independent spiking, and abnormal background activity in the unaffected hemisphere. Although they do not exclude a patient from hemispherectomy, the following abnormalities in the “unaffected” hemisphere may denote an unfavorable outcome: sporadic epileptiform discharges, abnormal secondary or independent EEG findings, and nonepileptiform abnormalities. This is especially true if independent interictal sharp wave activity is observed in the unaffected hemisphere.3,8 The anatomical details gleaned from preoperative imaging to assess the feasibility of hemispherectomy include the following: ventricular size, shape and depth of sylvian fissure, location of the circular sulcus, displacement of the normal landmarks, thickness of the corpus callosum, surface anatomy, presence of parenchymal atrophy, and abnormal vasculature. It has been noted that the presence of atrophy in the ipsilateral cerebral peduncle and medulla helps to predict a lower risk for postoperative worsening.3 On metabolic studies, confinement of the hypometabolic and epileptogenic areas to the damaged hemisphere has been shown to be a good predictor of outcome. By the same token, if the hypometabolic areas spread into the normal hemisphere, this is an indicator of more extensive and perhaps bilateral involvement.3 The ideal time for surgical intervention is debated. The goal of surgery is to protect the brain from the harmful effects of seizures and antiepileptic drugs. These effects on the immature brain, as well as the plasticity of the younger brain, argue for earlier surgery. After the sixth to eighth year, the ability of the dominant hemisphere to acquire language declines, which further supports earlier surgery. Additionally, the shorter the time between seizure onset and surgery, the higher the success rate, and earlier seizure control optimizes psychosocial development.3,22 As described before, there has been an evolution from anatomical hemispherectomy to functional hemispherectomy to hemispherotomy. The difference in terminology reflects the amount of cerebral cortex resected. The anatomical hemispherectomy resects the largest amount of cortex, whereas the functional hemispherectomy combines a partial resection with a partial disconnection. Finally, the hemispherotomy resects a small amount of cortex and maximizes disconnection.8 For a description of the anatomical hemispherectomy, the reader is referred to the article of Di Rocco et al.11 A question mark–type or a T-shaped skin incision can be used. An osteoplastic craniotomy can be fashioned, or a myocutaneous skin flap can be used with a standard craniotomy. The bone flap needs to be placed close to the sagittal sinus. The hemispherectomy is initiated by exposing the distal internal cerebral artery. The middle cerebral and anterior cerebral arteries are clipped and divided. Attention is then paid to the interhemispheric cleft. The medial surface of the hemisphere is retracted, and the bridging cortical veins are divided to reach the corpus callosum. The ipsilateral pericallosal artery is divided, and the callosotomy is performed from the genu to the splenium. Once the lateral ventricle is opened, the foramen of Monro is plugged. A transventricular ependymal incision lateral and anterior to the basal ganglia is made to detach the frontal lobe from the basal ganglia. The frontal lobectomy is completed by extending the ependymal incision up to the sylvian fissure and then continuing the incision from the fissure to the midline. Next, the parietal, occipital, and temporal lobes are disconnected. The posterior cerebral artery is divided at the level of its P3 segment. The temporal stem is divided by extending the previously created ependymal incision posteriorly to the trigone area and temporal horn. After coagulation of the bridging veins, the parieto-occipital lobe is freed with the temporal lobe and can be removed en bloc or in piecemeal fashion. An amygdalohippocampectomy is performed with subpial dissection or aspiration. The basal ganglia are left in situ unless they are felt to be involved in the cortical malformation, with a risk for recurrent seizures.11 As described previously, the Oxford modification consists of plugging the foramen of Monro and tacking the dura to the tentorium, falx, and floor of the middle cranial fossa to decrease the subdural space.7 As well, the Peacock addition includes routine subdural drainage with placement of a shunt in the early postoperative period.10 Rasmussen’s functional hemispherectomy was the first attempt at subtotal removal with disconnection. This consists of temporal lobectomy, resection of the central region, and disconnection of the residual frontal and parieto-occipital lobes from the remaining brain, with preservation of as many arteries and veins as possible. The insular cortex is preserved unless corticography has indicated spiking, in which case a subpial resection is performed.9 The goal is to provide functional disconnection while preserving a large part of the brain to avoid the complications of a large surgical cavity.3 The term hemispherotomy was introduced by Delalande et al in 1992 to describe minimal brain resection with maximal disconnection for intractable epilepsy.15 These procedures can be performed with a vertical or a lateral approach. Delalande and colleagues described a vertical approach. A linear transverse skin incision is made to allow a small parasagittal frontoparietal craniotomy that is based two-thirds posterior to the coronal suture and one-third anterior. A corticectomy is made to enter the lateral ventricle. A callosotomy is performed, and the posterior hippocampal disconnection is made by transecting the posterior column of the fornix at the level of the ventricular trigone. The corona radiata is transected to reach the temporal horn. An incision from the gyrus rectus to the anterior temporal horn creates the anterior disconnection.14,15,25 In 2001, Danielpour et al modified this technique by starting the vertical approach interhemispherically.3,26 The lateral approach was described similarly by Villemure and Mascott and by Schramm et al in 1995.16,27 Schramm et al describe it as starting with a hippocampectomy or a hippocampectomy with an anterior temporal lobectomy. After this, deafferentation of the white matter of the temporal, occipital, parietal, and frontal lobes is performed. This can be done with a transcortical transventricular approach along the outline of the lateral ventricle. The callosotomy is performed transventricularly. A small portion of the supra-insular cortex remains, as well as the insular cortex. However, as a modification, the insular cortex can be resected. Another modification allows the entire transventricular deafferentation to be performed through a sylvian keyhole. Villemure and Mascott described the technique of lateral peri-insular hemispherotomy in 1995. The authors stated it could be viewed as a “radical hemispheric tractotomy” resulting in a completely disconnected hemisphere.27 The craniotomy is based over the circular sulcus and the ventricular system. The peri-insular hemispherotomy has three major surgical stages: supra-insular window, infra-insular window, and insula. In the supra-insular window, the frontoparietal opercular cortex is resected, followed by the transection of the corona radiata and the transventricular callosotomy. The posterior hippocampectomy is completed, and the frontobasal disconnection completes the supra-insular window. The infra-insular window starts with the resection of the temporal opercular cortex, followed by the transection of the temporal stem. Next, the amygdala is resected, and the anterior hippocampectomy is completed. Finally, the insular stage consists of subpial aspiration of the insula or disconnection by incising at the level of the claustrum or extreme capsule.28 There have been several modifications to the peri-insular hemispherotomy. The universal steps are disruption of the internal capsule and corona radiata, resection of the medial temporal structures, transventricular callosotomy, and disruption of the horizontal fibers.29 Shimizu and Maehara introduced the transopercular hemispherotomy.34 The upper half of the insula is exposed through a transsylvian approach. The arteries of the insula are coagulated and divided. Next, the frontoparietal operculum and the upper half of the insula are resected en bloc. This creates a large cavity for which the disconnection and medial temporal lobe structures occurs.25,29 Comair used the transsylvian approach to perform the peri-insular disconnection. This involved a vertical incision from the insula to the temporal horn, thus allowing removal of the mesial temporal lobe structures. As well, a cortical incision is made to perform the callosotomy.29 Kanev et al used ultrasound to perform hemispheric disconnection without violating the ventricular cavity.30 In 2004, Cook et al described the modified lateral hemispherotomy. The goal was to reduce the blood loss seen with anatomical hemispherectomy and to decrease the reoperation rate seen with the Rasmussen functional hemispherectomy. It was also postulated that it would be key for children with small or malformed ventricles. This procedure creates a working space around the ventricles by removing most of the thalamus, basal ganglia, caudate nucleus, and other associated deep hemispheric structures. These deep structures were felt to be the cause of recurrent seizures that hinder the effectiveness of functional hemispherotomy.31 Other recommendations have been made to limit the size of the insular window to maintain as many middle cerebral artery branches as possible.29,32 At The Hospital for Sick Children, we have been using a modified peri-insular hemispherotomy approach. The patient is secured in a Sugita head holder (Mizuho, Tokyo, Japan) or a horseshoe head rest if very young. Neuronavigation is used to assist in marking the skin incision as well as localizing the ventricle. A question mark–type incision is based over the insula. A frontoparietotemporal craniotomy based on the insula is created (▶ Fig. 75.3). The dura is opened in a curvilinear fashion with spokes radiating superiorly, inferiorly, and posteriorly. The supra-insular window is created by resecting the frontoparietal opercular cortex down to the insular pial bank. Next, the infra-insular window is fashioned similarly to resect the temporal opercular cortex. Care is taken to preserve as many branches of the middle cerebral artery as possible to avoid remote infarcts or ischemic changes (▶ Fig. 75.4). Fig. 75.3 Artist’s rendering of the outline of a craniotomy for exposure of the supra- and infra-insular windows.
75.1 History
75.1.1 Anatomical Hemispherectomy
75.1.2 Functional Hemispherectomy
75.1.3 Hemispherotomy
75.1.4 Hemidecortication
75.2 Neurosurgical Procedure
75.2.1 Indications for Surgery
Sturge-Weber Syndrome
Cortical Dysplasia
Hemimegalencephaly
Rasmussen Syndrome
Porencephalic Cyst
Hemiconvulsion–Hemiplegia–Epilepsy Syndrome
75.2.2 Preoperative Planning
75.2.3 Timing of Surgery
75.2.4 Techniques of Hemispherectomy
Anatomical Hemispherectomy
Functional Hemispherectomy
Hemispherotomy
Hemispherectomy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








