Hippocampal Sclerosis
Gary W. Mathern
Charles L. Wilson
Heinz Beck
Introduction
Hippocampal sclerosis is the most frequent pathologic finding in patients with temporal lobe epilepsy undergoing resective neurosurgery, and its association with epilepsy has been recognized since the early 1800s. This chapter will focus on the following: The first section reviews the important historical literature to introduce the pathology and highlight some of the clinical controversies that often first arose years ago that are still argued about today.189 The middle section describes in vivo electrophysiologic findings associated with hippocampal sclerosis, with special emphasis on newly identified fast ripples that may be a surrogate marker of epileptogenesis. The final section highlights recent molecular studies that are beginning to identify possible epileptogenic mechanisms in hippocampal sclerosis related to changes in synaptic circuits, postsynaptic receptors, and intrinsic membrane properties. Because of the extensive literature, this chapter emphasizes findings mainly from human studies. Relevant studies related to animal models of temporal lobe epilepsy can be found in Chapters 36 and 40 and in other texts.163
Early History and Histopathologic Description of Hippocampal Sclerosis
The literature describing the clinicopathologic relationship between seizures and hippocampal pathology spans more than 150 years. Early studies were limited to autopsy material and concentrated on determining if individuals with seizures of any type showed cerebral and hippocampal pathology. It was not until autopsy studies in the 1930s and correlative surgical-pathology studies in the 1960s that the mesial temporal lobe epilepsy syndrome was linked to severe neuronal loss in a characteristic pattern, referred to in this chapter as hippocampal sclerosis. This clinicopathologic association has been complicated by a literature that uses numerous names for the hippocampal pathology found in individuals with epilepsy. Understanding the historical use of these terms in the context of the different clinicopathologic studies is perhaps the best way to comprehend what constitutes hippocampal sclerosis and the relationship of this pathologic substrate with temporal lobe epilepsy.
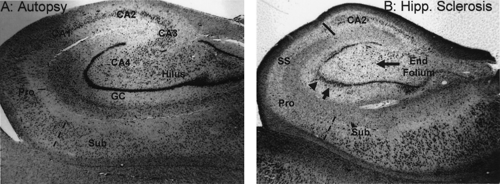
Early autopsy studies frequently observed hippocampal damage in patients with different types of epilepsy. At first, the term sclerosis referred to the gross macroscopic features of a hard shrunken hippocampus, first described by Bouchet and Cazauvielh in 1825,31 and in other autopsy studies of that era of individuals with chronic epilepsy.141,160 Probably the first microscopic description of hippocampal sclerosis was by Sommer in 1880,178 and his case study illustrates several important clinicopathologic features of temporal lobe epilepsy that are still relevant today. The patient was a 25-year-old man with what were described as two to six “petit mal” attacks each day, and several “complete” seizures each week. As part of his epileptic syndrome, he had vivid hallucinations in which God told him he could fly, and once, as proof of his belief, he jumped from a roof. He survived the fall only to die some years later of a systemic infection. Sommer observed that the only cerebral pathology found at autopsy involved the hippocampus, and using a microscope he noted a unique pattern of neuron loss. Specifically, the pyramidal neurons of the Ammon horn were largely destroyed, especially in the portion of the hippocampus that Lorente de Nó106 would later label as CA1 and prosubiculum. Neuron loss in CA1 is such a consistent finding in hippocampal sclerosis that this region is often referred to as the Sommer sector (see SS in Fig. 1B). Furthermore, Sommer described other hippocampal damage involving the granule cells and hilar neurons of the fascia dentate.
Sommer’s other historical contribution, in addition to the earliest microscopic description, was his interpretation that there must be a pathologic relationship between hippocampal damage and clinical seizure symptoms. He reasoned that the hippocampus was probably the initial site for seizure generation involving a prodrome of abnormal sensory phenomena or illusions. This is probably the first time that hippocampal pathology was associated with clinical features of what would probably be recognized today as mesial temporal lobe epilepsy.
The other important historical figure of the 1800s was Bratz.36 His contribution was a detailed histologic description of hippocampal sclerosis and the observation that not all seizure types were associated with hippocampal pathology. He reported pathologic findings in the brain from 50 autopsy specimens of patients with antemortem chronic seizures associated with various etiologies common in his day, such as syphilis and cysticercosis. Bratz found hippocampal sclerosis in 25 (50%) specimens. His 1899 microscopic observations were remarkably accurate, and neuropathologists today would use the same histopathologic criteria to define hippocampal sclerosis (Fig. 1). Bratz noted that there was severe pyramidal neuron loss and gliosis throughout the hippocampus, especially in the Sommer sector of the Ammon horn. In addition, he noted that subicular neurons were not as depleted and that a fairly sharp boundary separated the profound prosubiculum neuron loss from the relatively preserved subiculum (Fig. 1B, dashed line). Within the remainder of the hippocampus, there was a second area of severe damage involving neurons between the granule cell blades. This area, later termed the end folium by Margerison and Corsellis,111 included the CA4 pyramids and hilar neurons as described by Lorente de Nó.106 By comparison, fascia dentate granule cells were only partially destroyed. In contrast to the severe neuron loss in the Sommer sector and the end folium, pyramidal neurons in CA3 and, especially, CA2 seemed to be more “resistant” to injury (therefore termed the resistant sector).
Bratz’s histopathologic description of hippocampal sclerosis is different from the hippocampal neuronal injury associated with other cerebral diseases. For example, neuron loss
related to chronic liver disease or hypoxia-ischemia involves the entire hippocampus, including CA2, along with the subiculum and parahippocampal gyrus. Hence, neuron loss and gliosis by themselves are insufficient histopathologic criteria for the diagnosis of hippocampal sclerosis. This explains the confusion that has arisen from claims by some authors that hippocampal sclerosis can be found in conditions without seizures, such as Alzheimer disease.1,84 Thus, the histopathologic diagnosis of hippocampal sclerosis should be restricted to specimens that display the microscopic pattern of selective neuron loss as originally described by Bratz.
related to chronic liver disease or hypoxia-ischemia involves the entire hippocampus, including CA2, along with the subiculum and parahippocampal gyrus. Hence, neuron loss and gliosis by themselves are insufficient histopathologic criteria for the diagnosis of hippocampal sclerosis. This explains the confusion that has arisen from claims by some authors that hippocampal sclerosis can be found in conditions without seizures, such as Alzheimer disease.1,84 Thus, the histopathologic diagnosis of hippocampal sclerosis should be restricted to specimens that display the microscopic pattern of selective neuron loss as originally described by Bratz.
Bratz also noted that the neuronal loss in hippocampal sclerosis appeared old and chronic. Because hippocampal sclerosis was found in only half of the autopsy specimens of patients with epilepsy, he reasoned that this pattern of damage was not the result of repeated seizures. Instead, he suggested that hippocampal sclerosis probably generated certain types of seizures, similar to the conclusion of Sommer.178 Bratz’s final contribution was his observation that many of the patients with hippocampal sclerosis had clinical histories involving early childhood convulsions. This finding would resurface about 60 years later in clinicopathologic studies of surgical patients with mesial temporal lobe epilepsy, and the nearly continuous debate since then about the pathogenesis of hippocampal sclerosis.
Clinicopathologic Studies of Patients with Temporal Lobe Epilepsy
The studies of the 1800s confirmed an association between seizures and hippocampal pathology, but the link between hippocampal sclerosis with psychomotor and complex partial temporal lobe seizures took several more decades to confirm. The clinical autopsy study of Stauder187 was probably the first. He studied 53 patients with chronic epilepsy to determine whether hippocampal sclerosis was associated with those ictal symptoms and signs that he was convinced could only be attributed to temporal lobe seizures, such as olfactory and gustatory auras. Autopsy cases were separated into three clinical groups with definite, probable, or no ictal temporal lobe symptoms by clinical description. Of the 36 hippocampal sclerosis cases at autopsy, 33 (92%) had a history of definite or probable temporal lobe seizures. By contrast, of the 17 cases without sclerosis, only two (12%) showed only probable (not definite) temporal lobe seizure symptoms and 15 (88%) had none of his defined clinical signs. These results linked antemortem temporal lobe seizure symptoms with hippocampal sclerosis at autopsy.
In another famous autopsy study from the 1960s, Margerison and Corsellis111 examined pathology results in 55 patients with epilepsy and found a clinicopathologic association between antemortem temporal lobe seizures using clinical and electroencephalogram (EEG) criteria and postmortem hippocampal sclerosis. This study is often cited in the literature, and the reader should be aware of this study’s design, findings, and limitations. For example, their patient population is somewhat different than contemporary surgical case series of temporal lobe epilepsy patients. The patients resided in two long-term care hospitals in London because of severe mental and physical handicaps. Fifteen (27%) patients had chronic motor paralysis; in 17 (31%) the first habitual seizure was before the age of 1 year, and in 20 (36%) there were other cerebral cortical abnormalities such as congenital brain malformations, evidence of cerebral trauma, or old infections. Surgical patients with temporal lobe epilepsy typically have a lower incidence of paralysis and cerebral malformations, and the first habitual seizure usually begins around the age of 10 years.114 Margerison and Corsellis found that most of their patients regularly experienced generalized convulsions and only a proportion of the time had temporal lobe seizures. Margerison used clinical characteristics (n = 26; 47%) or interictal scalp EEG (n = 33; 60%) to identify those cases that in addition to generalized seizures, also probably had temporal lobe convul-sions.
Table 1 Autopsy-based Comparison of Qualitative Hippocampal Pathology in Patients with Intractable Seizures, Including Temporal Lobe Epilepsy | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
At autopsy, Corsellis defined two types of hippocampal damage. The first consisted of classic “Ammon horn sclerosis”
and the second was a new entity characterized by neuron loss limited only to the end folium. Corsellis introduced the term hippocampal sclerosis and defined it as an inclusive term that included both Ammon horn sclerosis and end folium sclerosis. (As a reminder, this chapter uses the term hippocampal sclerosis only to refer to what Corsellis considered classic Ammon horn sclerosis.) As shown in Table 1, hippocampal sclerosis (Ammon horn sclerosis) was found in 58% of autopsy specimens of patients with clinical or EEG criteria for temporal lobe seizures. By contrast, 60% of patients without clinical or EEG criteria for temporal lobe epilepsy had no hippocampal pathology (Chi-square; p <0.005). The incidence of end folium sclerosis averaged 25% for all patients with epilepsy, and was not associated with temporal lobe seizures (p <0.25). Hence, as in Stauder’s 1936 study,187 hippocampal sclerosis at autopsy was linked specifically to clinical and EEG characteristics of temporal lobe epilepsy. End folium sclerosis, however, was associated with repeated generalized seizures and was not a marker of temporal lobe epilepsy, as has been suggested by some authors.177
and the second was a new entity characterized by neuron loss limited only to the end folium. Corsellis introduced the term hippocampal sclerosis and defined it as an inclusive term that included both Ammon horn sclerosis and end folium sclerosis. (As a reminder, this chapter uses the term hippocampal sclerosis only to refer to what Corsellis considered classic Ammon horn sclerosis.) As shown in Table 1, hippocampal sclerosis (Ammon horn sclerosis) was found in 58% of autopsy specimens of patients with clinical or EEG criteria for temporal lobe seizures. By contrast, 60% of patients without clinical or EEG criteria for temporal lobe epilepsy had no hippocampal pathology (Chi-square; p <0.005). The incidence of end folium sclerosis averaged 25% for all patients with epilepsy, and was not associated with temporal lobe seizures (p <0.25). Hence, as in Stauder’s 1936 study,187 hippocampal sclerosis at autopsy was linked specifically to clinical and EEG characteristics of temporal lobe epilepsy. End folium sclerosis, however, was associated with repeated generalized seizures and was not a marker of temporal lobe epilepsy, as has been suggested by some authors.177
In addition to hippocampal neuron injury, Margerison and Corsellis found that there could be damage to other cerebral brain areas. For example, in their 22 cases of hippocampal sclerosis, additional damage was noted in the amygdala (64%), thalamus (50%), and neocortex (27%). Such findings are not limited to autopsy studies of patients with temporal lobe epilepsy. Falconer et al.67 found pathologic evidence for injury to the amygdala and white matter in an unspecified number of en bloc temporal lobe surgical specimens from patients with temporal lobe epilepsy and hippocampal sclerosis. These authors proposed the term mesial temporal sclerosis to indicate damage to the hippocampus and other mesial temporal sites. Recent magnetic resonance imaging (MRI) studies confirm the original autopsy pathology studies by finding evidence for extrahippocampal signal changes in a proportion of patients with temporal lobe epilepsy.19,48,145,208 Thus, while the traditional focus has been on hippocampal sclerosis as the probable site that generates chronic seizures, many patients will also demonstrate extrahippocampal injury. Such findings are germane to understand the pathogenesis of hippocampal sclerosis and how this pathology contributes to the development of seizures, and in deciding the extent of surgical resection in order to best achieve seizure control.
The Asymmetric Nature of Hippocampal Injury in Temporal Lobe Epilepsy
Autopsy and surgical studies support the idea that patients with intractable temporal lobe epilepsy frequently have bilateral hippocampal damage. However, the amount of damage is usually asymmetric, with one side showing hippocampal sclerosis and the other side milder forms of neuron loss. The best evidence comes from the study of Margerison and Corsellis,111 where of the 22 patients with EEG criteria for temporal lobe epilepsy and hippocampal sclerosis, the sclerosis was unilateral in 90% and bilateral in 10%. Similar results were reported by Sano and Malamud168 in another autopsy study of 29 patients with antemortem ictal “psychic phenomena.” Bilateral hippocampal damage was noted in 11 cases (39%). Meencke and Veith138 reported on results from 650 autopsy cases of patients with chronic epilepsy. They found hippocampal sclerosis in 198 (30.5%) and in 56% of these, the findings were bilateral but asymmetric. Similar results have been reported in the limited number of surgically treated temporal lobe epilepsy patients who later died and in whom the other hippocampus became available for study.6,126 In agreement with the pathology studies, brain MRI findings have shown that many patients with temporal lobe epilepsy have abnormal signal changes contralateral to the atrophic epileptogenic hippocampus.174,198 Thus, the available data support the concept that while hippocampal damage is often bilateral in patients with temporal lobe epilepsy, most of the time hippocampal sclerosis is unilateral and coincides with the epileptogenic focus.
Pathogenesis of Hippocampal Sclerosis
The pathologic origins of hippocampal sclerosis have been debated, often contentiously, for over 60 years, and center on whether neuron loss is the “cause” or “consequence” of
repeated temporal lobe seizures. Despite the remarkable astute clinicopathologic observations of Sommer178 and Bratz36 supporting the hypothesis that hippocampal sclerosis represented an area of chronic damage and gliosis that probably generated seizures, Spielmeyer,183 Scholz,172 Peiffer,159 and more recently Sutula and Pitkanen189 have argued that hippocampal injury is the consequence of repeated seizures. As will be detailed below, the correct answer probably lies somewhere in between.
repeated temporal lobe seizures. Despite the remarkable astute clinicopathologic observations of Sommer178 and Bratz36 supporting the hypothesis that hippocampal sclerosis represented an area of chronic damage and gliosis that probably generated seizures, Spielmeyer,183 Scholz,172 Peiffer,159 and more recently Sutula and Pitkanen189 have argued that hippocampal injury is the consequence of repeated seizures. As will be detailed below, the correct answer probably lies somewhere in between.
Earnest debate of the pathogenesis of hippocampal sclerosis began in the 1950s when surgical specimens became available from patients with temporal lobe epilepsy. Probably the first “modern” concept regarding pathogenesis was that of Earle et al.60 They examined 157 of Penfield’s temporal lobe resections and found macroscopic pathologic abnormalities in 100, ranging from focal gyral toughness to atrophy of the entire lobe. It should be noted that for many years Penfield did not routinely remove the hippocampus en bloc for histopathologic examination. Earl et al.60 suggested that the most likely explanation as to the “cause” of hippocampal sclerosis was transtentorial herniation of the mesial temporal lobe during a difficult delivery or as a result of birth anoxia with secondary brain swelling. They proposed that in herniating across the tentorium, the mesial temporal lobe compressed the adjacent posterior cerebral and anterior choroidal arteries to generate an ischemic lesion they termed incisural sclerosis. With time, this brain lesion “ripened” into an epileptic focus. Of interest, however, is that only 10% of their patients had a difficult birth history.
For the next 10 years, birth injury was considered to be the pathogenic etiology for hippocampal sclerosis, and this concept was initially supported by Falconer et al. in the United Kingdom.43,140 However, after reviewing their first 100 surgical cases in the early 1960s, Falconer began to realize that there was more than one possible clinical factor associated with hippocampal sclerosis. Of 47 cases of hippocampal sclerosis, a history of difficult birth, early childhood convulsions, or head injury was noted in 42 (89%). Of these, childhood seizures were the most common predisposing factor. Falconer et al. concluded that there was a strong association between a clinical history of childhood seizures, especially febrile seizures, and the finding of hippocampal sclerosis at surgery,39,67,68 and hypothesized that early seizures are a cause of hippocampal sclerosis. This idea has subsequently been referred to as Meyer’s hypothesis.139
This concept, however, has been challenged by epidemiologic studies showing that the risk of temporal lobe epilepsy after febrile convulsions is very low.3,42,149 Beginning in the mid-1990s, the UCLA group readdressed Falconer’s hypothesis in clinicopathologic studies of a large series of surgical patients with temporal lobe epilepsy.118,121,125 By expanding the concept of potential brain insults to include any significant medical event likely to injure the brain that occurred before the onset of epilepsy, the authors found that initial precipitating injuries were strongly linked to hippocampal sclerosis in surgical cases (Fig. 2A). However, initial precipitating injuries, while most likely to occur under age 5 years, were not restricted to a young age or to febrile convulsions. In fact, studies of hippocampi removed from pediatric patients with frequent nontemporal lobe seizures generally find only limited hippocampal neuron loss, an observation that supports the notion that childhood seizures, by themselves, do not lead to hippocampal sclerosis.121,123 Thus, the UCLA data supported previous arguments that hippocampal sclerosis predates the onset of epilepsy, is associated with some significant brain injury not necessarily linked with seizures or an early age, and is probably a cause of temporal lobe epilepsy. However, the UCLA group also found that seizure durations of 15 years or more were associated with progressive hippocampal neuron loss in all subfields of the hippocampus, and this occurred in temporal lobe epilepsy patients with or without hippocampal sclerosis (Fig. 2B). In other words, repeated seizures over time are associated with hippocampal neuron loss, but the damage is throughout the hippocampus and not in the selective pattern consistent with sclerosis. Recent neuroimaging and other studies have supported this conclusion.189 Thus, the pathogenesis of most cases of hippocampal sclerosis appears to be from some antecedent brain injury (thus acquired), but there is also progressive neuron loss with longer seizure durations. The latter finding may impact the decision as to when to refer patients with temporal lobe epilepsy for surgery.
Dual Pathology
Surgical patients with temporal lobe epilepsy sometimes have more than one lesion or area of injury within the temporal lobe. This is termed dual pathology, and it appears to be more common in younger patients with temporal lobe epilepsy.144 It has been difficult to interpret these studies because the definition of what constitutes a “second” pathology is sometimes vague, and it may also be unclear if both abnormalities are epileptogenic. For example, Babb and Brown7 found a 13% incidence of dual pathology when the other pathology was defined as a macroscopic mass lesion. Levesque et al.,96 using the same UCLA database, included microscopic lesions, such as heterotopias, and found dual pathology in 30% of temporal lobe resections. Other authors have reported rates of dual pathology ranging from 3% to 95% depending on the definition of a second pathology.5,88,190 Thus, a variable percentage of temporal lobe epilepsy patients will have hippocampal sclerosis plus some other histopathologic finding in the surgical specimen, such as an increase in heterotopic neurons in the subcortical white matter, but whether that second pathology contributes to seizure generation remains unclear despite recent attempts using intracranial EEG recordings.69,86
In Vivo Electrophysiologic Studies of Hippocampal Sclerosis
Intraoperative electrophysiologic recordings in the form of electrocorticography (ECoG) were the original source of functional data used to localize interictal spikes (see also Chapter 172). Histologic changes such as gliosis and neuronal loss in the resected tissue often correlate with interictal spikes and other abnormalities in the ECoG. A limitation of ECoG is that the activity comes from the cortical surface, not from deep structures like the hippocampus. Early attempts to examine the properties of the “epileptic neuron” were based on single unit recordings from the lateral temporal or frontal cortex carried out in the late 1960s and early 1970s.41,165 In a slightly later study, Wyler et al.207 sought to evaluate cellular discharges during intracranial recordings by identifying single unit burst discharge patterns that accompanied interictal spikes, and described synchronization of single unit discharges with one another and with local and surface field potentials during occasional intraoperative ictal events.
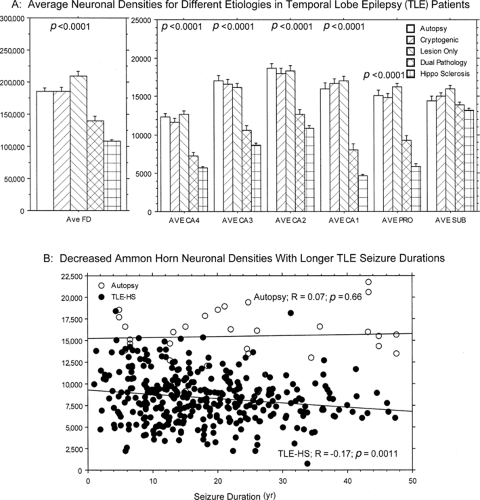 FIGURE 2. A: Bar graphs showing the mean (± SEM) neuron densities for the hippocampal subfields as labeled in FIGURE 1A for different etiology subgroups of temporal lobe epilepsy patients. Cryptogenic cases are those without magnetic resonance imaging (MRI) or histopathology-identified epileptogenic lesions. Lesion-only cases had an MRI-identified mass, such as a tumor or area of cortical dysplasia without hippocampal sclerosis. Dual pathology represents cases with hippocampal sclerosis and a mass lesion outside the hippocampus. An initial precipitating injury (IPI) was noted in 87% of patients with hippocampal sclerosis alone compared with 54% for those with dual pathology and 23% for those with cryptogenic temporal lobe epilepsy. ANOVA p values are illustrated above each subfield. Notice that neuronal densities for cryptogenic and lesion-only patients were similar to nonseizure autopsy cases, while dual pathology and hippocampal sclerosis patients with the higher incidence of IPIs had neuron loss in the profile expected with more damage in CA4, CA1, and prosubiculum. By comparison, damage in the subiculum for all cases was much less. B: Scatter plot illustrating progressive neuronal loss for the entire Ammon horn with longer duration of seizures in hippocampal sclerosis patients compared with autopsy cases. Notice that the progressive neuron loss in sclerosis patients required over 20 years of seizures and a large number of patients in the study group. |
Although McKhann et al.135 described use of ECoG re-corded directly from the hippocampus for determining the extent of hippocampal resection, intraoperative single neuron recordings from hippocampus are technically challenging and difficult to perform, particularly in terms of correlating such activity with hippocampal sclerosis. Perhaps the most direct intraoperative electrophysiologic correlates of hippocampal sclerosis were published by Rutecki et al.166 Prior to resection of hippocampal tissue, they stimulated the entorhinal cortex or alveus and recorded either from the surface of or from within the hippocampus. They compared evoked potentials recorded
in 16 patients with hippocampal sclerosis and eight without sclerosis. They found that the patients with hippocampal sclerosis showed simple monophasic or biphasic responses with long-onset latencies (mean, 21.9 msec), whereas those without sclerosis responded with complex, multiphasic potentials that had much shorter-onset latencies (11.8 msec). This is consistent with expectations for hippocampi with severe neuronal damage and gliosis characteristic of hippocampal sclerosis. However, such findings do not explain the epileptogenicity of hippocampal sclerosis, nor do they provide histologic identification of the position of the recording electrode in relation to hippocampal laminae. Of more promise are recent investigations using multiple contact microelectrodes with vertical spacing small enough to study the voltage depth profile of tissue in hippocampal sclerosis.192
in 16 patients with hippocampal sclerosis and eight without sclerosis. They found that the patients with hippocampal sclerosis showed simple monophasic or biphasic responses with long-onset latencies (mean, 21.9 msec), whereas those without sclerosis responded with complex, multiphasic potentials that had much shorter-onset latencies (11.8 msec). This is consistent with expectations for hippocampi with severe neuronal damage and gliosis characteristic of hippocampal sclerosis. However, such findings do not explain the epileptogenicity of hippocampal sclerosis, nor do they provide histologic identification of the position of the recording electrode in relation to hippocampal laminae. Of more promise are recent investigations using multiple contact microelectrodes with vertical spacing small enough to study the voltage depth profile of tissue in hippocampal sclerosis.192
Electrical Stimulation and Hippocampal Sclerosis
Intra- and extraoperative electrical stimulation has been used since the time of Penfield in the evaluation of epilepsy patients. Studies carried out in patients with depth electrodes have focused on the mental phenomena or memories evoked in awake subjects during trains of high-frequency hippocampal stimulation.17,73,75 However, electrical stimulation of mesial temporal structures, including hippocampus, has also provided localizing data based on evocation of the stereotyped auras or behavioral changes associated with a patient’s characteristic spontaneous seizures.23 Although the relationship between behavioral responses to stimulation and hippocampal sclerosis is unclear, hippocampal stimulation has provided evidence for both increased evoked potential thresholds and increased afterdischarge thresholds in sclerotic hippocampi compared with nonsclerotic tissue.45 In 74 patients with depth electrodes, single pulse stimulation was used to measure the functional intrinsic connectivity and the efferent and afferent connections of hippocampus with other mesial temporal and limbic structures.202 Latencies and conduction velocities of field potentials evoked by single pulses of electrical stimulation varied among the seven limbic sites studied, but the two mesial temporal structures that showed greatest connectivity with all other areas were the entorhinal cortex and the presubiculum. This finding is expected based on the known afferent and efferent pathways of the hippocampus. In a subsequent study, functional connections on the side of seizure onset were found to be significantly decreased within the entorhinal cortex, between the anterior and more posterior hippocampus, and between the hippocampus and amygdala.200 If one assumes that the preponderance of unilateral mesial temporal onsets in these patients were associated with the presence of sclerotic hippocampi, these results provide further support for reductions in neuronal network connectivity in hippocampal sclerosis.
Paired pulse stimulation has also been employed in evaluating excitability in the hippocampus.203 In 15 patients, paired pulse suppression in the perforant pathway was significantly greater in the epileptogenic hippocampus compared to the contralateral side. These results demonstrate that inhibition is maintained or even increased in the synaptically reorganized hippocampus in spite of the cell loss and gliosis characteristic of hippocampal sclerosis.
Microdialysis Studies in Hippocampal Sclerosis
In some surgical centers, electrophysiologic recording from depth electrodes has been accompanied by in vivo micro-dialysis.59,204 This has provided an opportunity to evaluate the release of glutamate and other neurotransmitters associated with hippocampal seizure activity. During and Spencer59 showed that glutamate release occurred not only during seizures, but also preceding seizure onset. Furthermore, the glutamate release was much greater on the side of seizure onset in patients with hippocampal sclerosis. They suggested that the high levels of glutamate occurring during seizures could reach neurotoxic levels and play a role in the progressive neuronal loss associated with hippocampal sclerosis (Fig. 2B). They also speculated that reuptake transporters were not functioning properly. This study also showed prominent γ-aminobutyric acid (GABA) release during seizures in the nonsclerotic hippocampus and less release on the side of hippocampal sclerosis. In another paper, During et al.58 showed that GABA release during K+depolarization was increased in the hippocampus of the epileptogenic temporal lobe, but there was no difference from baseline when the microdialysis perfusate was Ca+2 free. During et al. provided additional evidence suggesting that this GABA release was mediated by reverse transport, not synaptic release. Such findings are consistent with anatomic data showing changes in glutamate and GABA transporters.124 A more recent study by Cavus et al.,44 using zero flow measures of baseline amino acids, supports the conclusion that hippocampal sclerosis is associated with high lactate levels, a general reduction in the glutamate-glutamine cycle and glutamate uptake, which leads to increased glutamate levels and neurotoxicity (see Chapter 87).
Ictal and Interictal EEG Correlates of Hippocampal Sclerosis
The well-established association between histologic damage and seizure propensity has been clarified over the years by correlating the electrographic changes recorded using depth electrodes with various measures of neuronal loss. These include the presence of hippocampal sclerosis with focal ictal onsets or interictal spikes,64,102,197 the pattern of hippocampal pyramidal and granule cell loss correlated with the area of hippocampal ictal onset,8,12,180,181 cell density in sclerotic or atrophic hippocampus correlated with interhemispheric propagation time,99,101,179,182 or hippocampal thiopental EEG activation.100 The correlation of interictal spikes with hippocampal sclerosis is clearly state dependent, because interictal spikes are widespread during slow-wave sleep but may be focal during rapid eye movement (REM) sleep.103,109,110,167 Sensory-evoked or event-related potentials also have been considered a means of assessing hippocampal or mesial temporal damage associated with temporal lobe epilepsy.46,75,133,134,136,137
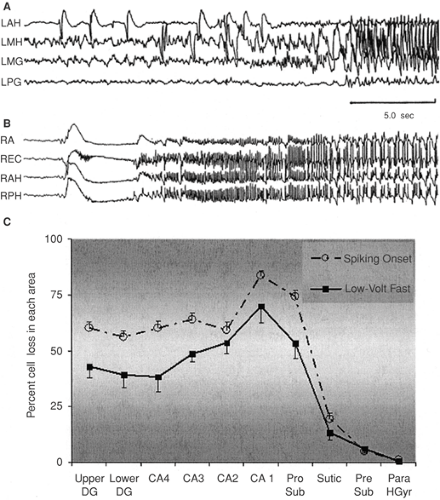 FIGURE 3. Unilateral recordings from hippocampus and adjacent structures in two different patients. A: A hypersynchronous spiking on-set. LAH, left anterior hippocampus; LMH, left middle hippocampus; LMG, left middle parahippocampal gyrus; LPG, left posterior parahippocampal gyrus. B: A low-voltage fast (LVF) onset. RA, right amygdala; REC, right entorhinal cortex; RAH, right anterior hippocampus; RPH, right posterior hippocampus. C: Mean percentage cell loss (± SEM), with LVF (14 patients) versus hypersynchronous (29 patients) on-sets. Notice that both groups have a neuronal loss profile consistent with hippocampal sclerosis with slightly less damage in those with the hypersynchronous spiking onset. Upper DG, upper blade of dentate gyrus; Lower DG, lower blade of dentate gyrus; CA1–4, hippocampal fields 1–4; Pro Sub, prosubiculum; Subic, subiculum; Pre Sub, presubiculum; Para HGyr, parahippocampal gyrus. All sites were significantly different at p <0.05 or better with the ex-ception of CA2, Subic, Pre Sub, and Para HGyr.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|