Hormone Changes in Epilepsy
Cynthia L. Harden
Cheryl A. Frye
Introduction
The relationship between hormones and seizures has long been recognized with the observation of prolactin elevation immediately following generalized seizures. Furthermore, this clinical phenomenon has been diagnostically useful. More recently, however, studies of the effects of seizures on endocrine function have largely focused on reproductive issues. The disruptive effects of seizures on reproductive endocrine functioning are not immediate, however, and appear to develop as a result of chronic dysregulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secondary to abnormalities in gonadotropin-releasing hormone (GnRH) from the hypothalamus. This chapter discusses evidence for effects of seizures on hormones and the clinical consequences of this relationship.
Effects of Epilepsy on Hormones in Animal Models
Stress-related Hormones
Stress produces an increase in circulating and brain concentrations of corticosteroids, most importantly cortisol and deoxyocorticosterone. Given that seizures themselves are stressful events, it is expected that stress hormones will increase with seizures. The hormones increased by stress have mixed proconvulsant and anticonvulsant effects. Both pregnenolone sulfate and dehydroepiandrosterone sulfate are increased by stress, and each has a proconvulsant effect in animal models. Deoxyocorticosterone is metabolized to allotetrahydrodeoxycorticosterone, however, which activates γ-aminobutyric acid A (GABAA) receptors. Therefore, seizures may initiate the release of hormones that have mixed effects on brain excitability.41 The GABAA receptors of hippocampal neurons in epileptic animals have diminished capacity for modulation by neuroactive steroids,35 which supports the presence of an interaction between seizures and neurosteroid activity in the brain.
One hypothesis for the etiology of West syndrome and its accompanying infantile spasms is related to the presence of abundant corticotrophin-releasing hormone (CRH) receptors in the brain normally present at the critical infantile time period. Dysfunction of the brain’s response to stress-induced CRH elevation triggers the syndrome and further explains the therapeutic response to a hormone that suppresses CRH production, adrenocorticotropic hormone (ACTH).8
Reproductive Hormones
Regulation of reproduction involves brain structures as part of a complex, multilocalized system termed the hypothalamic-pituitary-gonadal axis. A critical portion of the hypothalamic-pituitary-gonadal axis is the small and scattered population of GnRH-producing neurons within the diagonal band of Broca, the vasculosum of the lamina terminalis, and the preoptic area of the hypothalamus. When activated, these neurons secrete GnRH into the hypophyseal-portal vasculature, and this hormone then regulates the production and release of the two gonadotropins, LH and FSH. The synchronization and responsiveness of this hormonal system are essential for normal reproductive functioning.15
Seizures produce reproductive dysfunction in animal models of epilepsy, and these experiments provide further information about mechanisms of epilepsy-related reproductive dysfunction in humans. Kindling in the basolateral amygdala of intact rats produces reproductive alterations roughly analogous to polycystic ovary syndrome among women. Amygdala kindling disrupts ovarian cyclicity and produces cystic ovarian follicles, high E2 levels, and increased pituitary weights.13 The abnormalities in the target organs of the hypothalamic-pituitary-gonadal axis may be due to disruption of normal GnRH release in the hypothalamus due to seizure activity. The evidence for this is that GnRH fibers are reduced following pilocarpine-induced status epilepticus or focal application of kainic acid to the amygdala.1,16 These findings suggest that temporal limbic structures, such as the hippocampus and the amygdala, that are involved in seizures alter reproductive function via the effects on gonadotropin secretion.
Effects of Epilepsy on Hormones in Humans
Acute Changes in Hormones Following Seizures: Prolactin, Follicle-stimulating Hormone, and Luteinizing Hormone
Further evidence for an effect of seizures on hypothalamic activity is the long-observed elevation in prolactin that occurs after generalized tonic–clonic seizures. Like that of LH and FSH, control of prolactin release from the pituitary is also under hypothalamic control. Acute changes following seizures include increases in prolactin for 20 minutes and serum LH (in women and men) and FSH (in women) for 60 minutes following generalized tonic–clonic seizures.11,38,43 A recent, evidence-based report on the use of serum prolactin levels in diagnosing epileptic seizures recommended that an elevated prolactin within 10 to 20 minutes after a suspected event is a useful adjunct in differentiating generalized tonic–clonic or complex partial seizures in adults and older children from psychogenic nonepileptic seizures. Syncope may also elevate prolactin levels, however, limiting its diagnostic usefulness.9 Furthermore, prolactin levels cannot be reliably used for this purpose in infants or in status epilepticus.9
Effects of Epilepsy on Luteinizing Hormone Pulsatility; a Possible Cause of Hypogonadotropic Hypogonadism
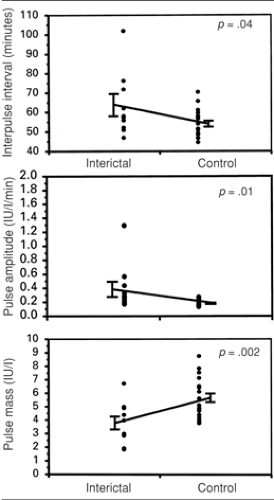
It is possible that the disruption in gonadotropin secretion produced by seizures, leading to a dysfunctional feedback system between reproductive hormones and LH and FSH secretion, is related to the increased incidence of reproductive endocrine disorders and infertility observed among those with epilepsy.7 Epilepsy itself, incorporating the subclinical activity of interictal spikes, can also disrupt the finely tuned, pulsatile secretion of LH. It has been shown that LH secretion can be disrupted by interictal spikes in men and women (Fig. 1).6,12,28,40 Recently, a differential effect of interictal and postictal effects on LH secretion in men with epilepsy was reported. The investigators reported that the circadian peak concentration of LH is delayed interictally compared to controls, and that burst amplitude is generally lower throughout the day. However, postictally, within 24 hours of a seizure, the peak concentrations of LH pulses were dispersed to a more random, rather than rhythmic pattern, with longer interburst interval.39 These findings offer even more refinement of the evidence for a negative effect of seizures and epilepsy on reproductive functioning and indicate that this sensitive system can be easily disrupted, with clinical consequences.
The absent or decreased ability of the hypothalamus to secrete GnRH or of the pituitary gland to secrete LH and FSH leads to hypogonadotropic hypogonadism, which is a failure of gonadal function due to inadequate stimulation by LH and FSH. This syndrome, or at least partial features of it, has been reported in both men and women with epilepsy.25,31,36 In one report, hypogonadotropic hypogonadism as defined by reduced gonadotropin release, loss of cyclicity, and/or infertility was present in 12% of women with temporal lobe epilepsy versus 1.5% in the general population.25
Polycystic Ovary Syndrome and Epilepsy
As an example of this dysfunctional feedback system, the incidence of polycystic ovary syndrome (PCOS), a form of hyperandrogenic chronic anovulation, which generates abnormal androgen levels and positive feedback at the level of the hypothalamus, ranges from 10% to 25% among women with temporal lobe epilepsy and from 4% to 6% in the general population.25 One possible cause of this mysterious syndrome is thought to be centrally mediated as a result of abnormalities in LH and FSH stimulation of the ovary. This abnormal hormonal environment results in multiple immature ovarian follicles, which form cysts and secrete testosterone rather than estrogen, which is secreted from a matured follicle. Therefore, PCOS may also be a consequence of a dysregulated hypothalamic-pituitary-gonadal axis.
Although valproate has been associated with PCOS, it is unclear whether it causes, exacerbates, or imitates PCOS because it can elevate androgens in both men and women (see also Chapters 108 and 198). Valproate likely increases androgens, at least in part, by inhibition of aromatase, the enzyme involved in conversion of testosterone to estrogen.19 In an evaluation of 93 women with focal epilepsy of long duration, PCOS (defined as elevated testosterone levels and oligomenorrhea or amenorrhea) occurred in 10.6%. No difference was found between women taking carbamazepine (n = 20; 10%), valproate (n = 18; 11.1%), or no antiepileptic drugs (AEDs) (n = 19; 10.5%).4 This study confirms the finding that PCOS is present at a higher-than-expected rate in women with epilepsy, but it does not support a clear association with valproate use.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree