Idiosyncratic Adverse Reactions
Munir Pirmohamed
Santiago Arroyo
Introduction
A perusal of the product information sheets for all antiepileptic drugs (AEDs) reveals a long list of adverse effects. Fortunately, the majority of these adverse effects are mild and predictable from the known pharmacology of the AEDs. These types of adverse reactions are termed type A (“augmented”) adverse reactions.81 They typically tend to be dose dependent, usually improve with reduction in dose, and tend to be identified early during the course of the development of the drug.
AEDs can also cause type B (“bizarre”) or idiosyncratic adverse reactions,81 which are the focus of this chapter. These can be severe and sometimes fatal; they cannot be predicted from the known pharmacology of the drugs. Typically, there is no clear relationship between the occurrence of the reaction and the dose administered to the patient. Nevertheless, for some idiosyncratic adverse reactions, there is evidence of some relationship to dose and/or the speed of titration; for example, the risk of phenytoin-induced rashes seems to be greater if the starting dose is high.16 Similarly, with lamotrigine, patients started on lower doses and slower titration schedule have a reduced risk of developing rashes.110
Idiosyncratic adverse reactions may be acute—occurring soon after the start of therapy—or chronic—occurring after prolonged therapy. They cannot be reproduced in animal models, and the only model in which they can be studied is the human.71 Idiosyncratic adverse reactions, as the name implies, cannot be predicted and tend to affect a minority of patients taking the drug; the risk factors for individual susceptibility in most cases have not been identified, and genetic factors are thought to be important, although the supportive evidence only exists for a minority of the reactions. Due to their infrequent occurrence, many idiosyncratic adverse reactions are usually not detected during the premarketing phases of drug development and are only identified after licensing when there is high market exposure of the drug. Statistically, this is not surprising given that 30,000 patients have to be exposed to have a 95% probability of detecting 1 case of an adverse reaction occurring at a frequency of 1 in 10,000.79
Detection of idiosyncratic adverse reactions therefore largely, although not exclusively, depends on postmarketing surveillance strategies, in particular spontaneous reporting systems such as the MEDWATCH scheme in the United States and the Yellow Card scheme in the United Kingdom. There are limitations of spontaneous reporting systems, however, the most important being the degree of underreporting: Less than 10% of serious adverse reactions are reported.89 This can lead to a substantial delay in detecting idiosyncratic adverse reactions after the drug is introduced onto the market. In some cases, certain idiosyncratic reactions, although frequent, might not be easily clinically demonstrable, leading to a substantial underreporting. For example, vigabatrin was introduced onto the U.K. market in 1989, but the first reports of visual field constriction appeared 8 years later in 1997,29 despite the fact that we now know that the prevalence of vigabatrin-induced visual field constriction is between 30% and 40%.22 In addition, data on adverse reactions submitted to the reporting systems are often incomplete and inadequate, being collected from different sources without expertise or standardization of terms and diagnostic criteria, making their evaluation more difficult.53
Idiosyncratic adverse reactions associated with AEDs can take many forms and can affect any body system (Table 1). In this chapter, we do not cover all of the reactions that have been reported with AEDs but focus on certain areas that we feel are perhaps the most important because of their prevalence, severity, or both.
Hypersensitivity Reactions
In this chapter, we use the term hypersensitivity to indicate a presumed allergic drug reaction, most commonly manifested as a maculopapular eruption. In some cases, the associated rash may be more severe, with the development of blisters and involvement of the mucous membranes, as in Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). In other cases, the reaction, with or without rash, may be associated with fever, eosinophilia, arthralgia, and internal organ involvement, a condition known as anticonvulsant hypersensitivity syndrome (AHS), also termed DRESS (drug rash with eosinophilia and systemic symptoms).
Maculopapular eruptions have been reported with most AEDs (Table 2).5 Fortunately, with most of the drugs, such reactions are relatively mild and infrequent, but they do represent a problem particularly with the aromatic AEDs (phenytoin, carbamazepine, and phenobarbitone) and lamotrigine, in which case they may become more serious with the development of SJS or TEN, or AHS. Maculopapular eruptions are relatively common, occurring in 5% to 15% of patients receiving aromatic AEDs16,88 and in 10% of patients on lamotrigine.40 These figures probably represent overestimates, however, because the frequency of rashes is lower with more modern dosing strategies, which start at a low dose and increase the dose slowly.63 AHS is less common; the risk within the first 60 days of a new prescription for either phenytoin or carbamazepine has been estimated to be 2.3 to 4.5 per 10,000 and 1 to 4.1 per 10,000, respectively.101 The incidence of SJS and TEN is not known; these conditions are relatively rare, however, with a global population incidence of 1 case per million per year.18 It is interesting to note that lamotrigine was associated with a higher risk of SJS, particularly in children (1 in 100 incidence), at the time of its introduction.62
Table 1 Idiosyncratic adverse reactions reported with antiepileptic drugs | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Table 2 Some Antiepileptic drugs reported to cause hypersensitivity reactions | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Pathogenesis
Hypersensitivity reactions caused by AEDs have an immune pathogenesis. Initially this was an assumption based on
clinical features (see later discussion) and the rapid recurrence of the reaction on rechallenge.84 However, more recently, direct evidence indicating that drug-specific T cells are involved in the pathogenesis of hypersensitivity reactions associated with phenytoin,59 carbamazepine,59,66 and lamotrigine67 has been provided. The exact antigens responsible for these reactions, however, remain elusive. Traditionally, it has been assumed that these drugs underwent bioactivation to toxic metabolites such as arene oxides, which then covalently bound to proteins and acted as haptens.7 According to this hapten hypothesis, the drug–protein conjugate would be recognized as being foreign and an immune response would result. More recently, it has also been suggested that AEDs may be able to stimulate an immune response through direct interaction with the T cell receptors in the absence of any covalent binding or antigen processing.78 However, such studies have been performed in vitro, and whether the same also occurs in vivo is unclear. It is of interest that it has also been suggested that activation of human herpesvirus 6 (HHV-6) may be responsible for propagating the severity of the hypersensitivity reaction.69 However, this has largely been shown in Japanese patients, and given the high frequency of background infection with HHV-6 (almost 100% of patients have been exposed to it), it is not clear whether this represents an epiphenomenon or is causal.
clinical features (see later discussion) and the rapid recurrence of the reaction on rechallenge.84 However, more recently, direct evidence indicating that drug-specific T cells are involved in the pathogenesis of hypersensitivity reactions associated with phenytoin,59 carbamazepine,59,66 and lamotrigine67 has been provided. The exact antigens responsible for these reactions, however, remain elusive. Traditionally, it has been assumed that these drugs underwent bioactivation to toxic metabolites such as arene oxides, which then covalently bound to proteins and acted as haptens.7 According to this hapten hypothesis, the drug–protein conjugate would be recognized as being foreign and an immune response would result. More recently, it has also been suggested that AEDs may be able to stimulate an immune response through direct interaction with the T cell receptors in the absence of any covalent binding or antigen processing.78 However, such studies have been performed in vitro, and whether the same also occurs in vivo is unclear. It is of interest that it has also been suggested that activation of human herpesvirus 6 (HHV-6) may be responsible for propagating the severity of the hypersensitivity reaction.69 However, this has largely been shown in Japanese patients, and given the high frequency of background infection with HHV-6 (almost 100% of patients have been exposed to it), it is not clear whether this represents an epiphenomenon or is causal.
Clinical Manifestations
The most common manifestation of a hypersensitivity reaction is a generalized maculopapular eruption (Fig. 1), which typically occurs within the first 3 months of therapy (with a peak in the first 6 weeks). These reactions are usually mild, are not accompanied by systemic symptoms, and resolve on drug discontinuation. Very occasionally, the rash may manifest as an urticarial reaction (FIGURE 1). The rash may be a prelude to the development of full-blown AHS. AHS is manifested by fever (100% of cases), rash (87%), eosinophilia (30%), and atypical lymphocytosis (6%). Internal organ involvement is also common, the liver being the most commonly affected (51%), followed by the hematologic system (23%), kidneys (11%), and lungs (9%).96 Additional clinical findings may include periorbital or facial edema, exudative tonsillitis, oral ulcers, strawberry tongue, hepatosplenomegaly, flu-like symptoms, myopathy, disseminated intravascular coagulopathy, and pharyngitis.94
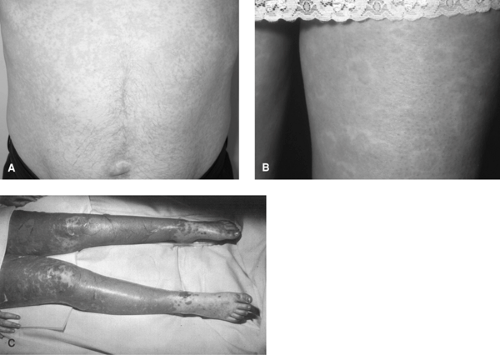 FIGURE 1. Cutaneous manifestations of antiepileptic drug hypersensitivity. A: Patient with phenytoin-induced maculopapular eruption. (Figure kindly provided by Dr. Gavin Wong, Liverpool, U.K.). B. Patient with carbamazepine-induced urticaria. (Figure kindly provided by Dr. Clodagh King, Liverpool, U.K.). C. Patient with carbamazepine-induced toxic epidermal necrolysis. (See the color insert.) |
Very occasionally, an erythematous rash may progress to a more severe, blistering condition such as SJS or TEN (FIGURE 1). SJS is characterized by blistering affecting <10% of the body surface area; whereas in TEN, >30% of the body surface area is blistered. Blistering between 10% and 30% is termed the SJS-TEN overlap syndrome.90 These conditions are characterized by the involvement of at least two mucous membranes, which is uncommon in AHS. Atypical target lesions may also be present in SJS but are absent in AHS. Lymphadenopathy is more common in AHS than in SJS.39
Predisposing Factors
The higher incidence of AHS reported in blacks may be a reflection of a higher incidence of epilepsy in this patient group.94 Chinese seem to have a higher predisposition
to develop SJS than whites. Consistent with this, a highly significant association of carbamazepine-induced SJS with HLA-B*1502 has recently been reported in Han Chinese.19 This does not seem to be relevant in whites, however, in whom AHS has been associated with major histocompatibility complex (MHC) polymorphisms residing in the ancestral haplotype 8.1.80
to develop SJS than whites. Consistent with this, a highly significant association of carbamazepine-induced SJS with HLA-B*1502 has recently been reported in Han Chinese.19 This does not seem to be relevant in whites, however, in whom AHS has been associated with major histocompatibility complex (MHC) polymorphisms residing in the ancestral haplotype 8.1.80
Management
The key to clinical management is the prompt recognition of the occurrence of the hypersensitivity and withdrawal of the offending drug. With mild maculopapular eruptions, this is often all that is needed, with the reaction improving within a few days. AHS is more severe and may require hospitalization. After withdrawal of the drug, patients may require symptomatic (e.g., antipyretics) and supportive therapy. Corticosteroids are often used, but there is no good randomized, controlled data on their effectiveness.5 Severe involvement of the internal organs may require treatment by specialists in the field. This is also certainly true of patients with SJS or TEN, in whom treatment in a specialist unit is vital. Nursing care, often in intensive care settings, and adequate topical management reduce associated morbidity and allow a more rapid reepithelialization of skin lesions.37 Many different therapies have been tried in patients with SJS/TEN, including corticosteroids, intravenous immunoglobulins, and plasmapheresis, but evidence of efficacy is either lacking or contradictory. This issue is beyond the scope of this chapter; see the article by Ghislain and Roujeau37 for more details. Patients with AHS usually improve over 2 to 3 weeks, but some patients may experience a flare of symptoms a few weeks after the initial improvement. Mortality of AHS, especially if the liver is involved, can be as high as 20%.94 Patients with SJS/TEN may take several weeks to improve; mortality is high at 5% for SJS and 30% for TEN.37
Prevention
Initiation of AED therapy at low doses and slow uptitration seems to be effective in reducing the incidence and severity of hypersensitivity with aromatic AEDs and with lamotrigine.5 Prompt discontinuation of the offending drug has also been shown to reduce progression to a more severe form of the reaction.35 There is a theoretical risk that sudden withdrawal of an AED may precipitate status epilepticus; however, this has not been borne out in clinical practice if the patient’s treatment is continued with an alternative agent.39 When the risk of withdrawal seizures is thought to be high, short-term benzodiazepines represent a suitable treatment option. In patients who have had a hypersensitivity reaction with an aromatic AED such as phenytoin, it is prudent to avoid another drug within the same class because of the risk of cross-sensitivity. In vitro, the risk has been estimated to be as high as 80%,96 but clinically it is probably lower, around 20%.83 Valproic acid is probably a safe alternative to aromatic AEDs in patients on monotherapy; lamotrigine, despite the fact that it does not demonstrate cross-reactivity with aromatic AEDs, is best avoided because rapid uptitration of the dose is contraindicated. In patients on polytherapy, other drugs such as levetiracetam and gabapentin may also be safe alternatives.
In terms of drug development, prevention of AED hypersensitivity has depended on the development of drugs that do not undergo hepatic metabolism such as levetiracetam, gabapentin, and vigabatrin. Hypersensitivity is not a recognized clinical problem with these drugs, although there have been occasional reports (Table 2). Oxcarbazepine, a keto analog of carbamazepine, is associated with a lower incidence
of hypersensitivity, with cross-reactivity being seen in 30% of patients.48
of hypersensitivity, with cross-reactivity being seen in 30% of patients.48
Liver Toxicity
Liver injury has been observed with many AEDs; its severity varies and can range from mild asymptomatic elevation of the liver enzymes to acute liver failure, which may require liver transplantation. The hepatotoxicity may be part of the spectrum of AHS, particularly with the aromatic AEDs. However, it can occur in isolation, in which case the pathogenesis may be immune mediated, for example, aromatic anticonvulsants, or due to metabolic idiosyncrasy, for example, with valproic acid.
Aromatic Anticonvulsants
Enzyme induction by these drugs can lead to an increase in γ-glutamyltransferase (γ-GT) and, to a lesser extent, in alkaline phosphatase (ALP). For instance, 64% and 14% of patients on carbamazepine had elevations of γ-GT and ALP, respectively.99 Phenytoin leads to asymptomatic elevation of γ-GT in almost 100% of recipients.3 In general, it is not necessary to stop the drug.
Mild elevation of serum transaminases is also commonly seen (22% with carbamazepine74), and this sometimes normalizes despite continuation of therapy.3 The relationship to more severe forms of liver dysfunction is unclear.
Clinically symptomatic hepatic injury is often part of AHS (see prior discussion), although the liver can be affected on its own.74,96 The exact incidence is unknown; an analysis of all adverse reactions to carbamazepine reported to the Swedish Regulatory Agency showed that liver disorders accounted for 10% of all reactions.7 The risk was estimated to be 16 cases per 100,000 treatment years. The time to onset of symptomatic hepatotoxicity is about 4 weeks with a range of 1 to 16 weeks.107 Clinically, liver involvement may be characterized by right upper quadrant abdominal pain and jaundice, which is seen in nearly one half of the patients with hepatitis.30 Biochemical abnormalities include an elevation of transaminases; however, about 30% of patients with carbamazepine-induced liver injury have a cholestatic pattern. Certainly, cholestasis seems to be more common with carbamazepine than with phenytoin. A prolonged rise in bilirubin levels similar to that seen in primary biliary cirrhosis is observed in patients with vanishing bile duct syndrome secondary to carbamazepine.31 In patients with hepatocellular necrosis, the rise in bilirubin levels reflects the severity of damage65 and may be accompanied by changes in clotting parameters. Histologically, granulomatous hepatitis is observed in up to three fourths of patients with carbamazepine-induced liver injury107 but is less common with phenytoin, which is more commonly characterized by hepatocellular injury accompanied by a prominent inflammatory infiltrate.64 Prognosis is worse in those with a predominantly hepatocellular pattern than in those with cholestatic injury.107
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





