Chapter 16 Implantation of Intrathecal Drug Delivery Systems
Indications
Implantation of an intrathecal drug delivery system is indicated for:
 Long-term infusion of baclofen for severe spasticity of spinal or cerebral origin that responds to baclofen
Long-term infusion of baclofen for severe spasticity of spinal or cerebral origin that responds to baclofenContraindications
Contraindications to implantation of an intrathecal drug delivery system are as follows:
Instrumentation
Figure 16–1 Various sizes of Tricumed intrathecal pumps (Tricumed Medizintechnik GmbH, Kiel, Germany).
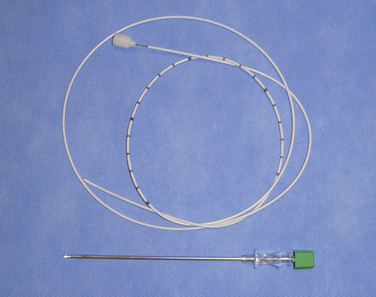
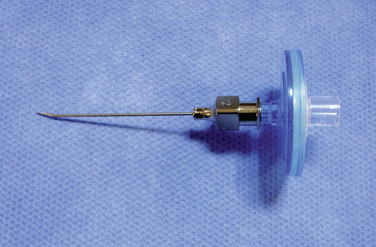
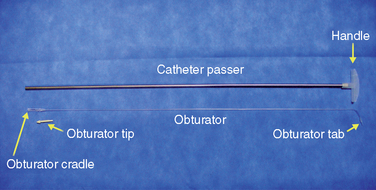
Figure 16–5 A catheter with a stylet (top) and a 15-gauge spinal needle (bottom) for spinal catheterization.
Drugs needed for implantation of an intrathecal drug delivery system are as follows:
 5 mL of preservative-free 0.9% normal saline (for flushing catheter access port when using the SynchroMed II Infusion System pump [Medtronic, Inc., Minneapolis, MN])
5 mL of preservative-free 0.9% normal saline (for flushing catheter access port when using the SynchroMed II Infusion System pump [Medtronic, Inc., Minneapolis, MN])Preoperative preparation
Physical examination should include the following actions:
 Nonsteroidal anti-inflammatory drug (NSAID), aspirin, and/or anticoagulant therapy should be stopped for 3 to 7 days before and after the procedure.
Nonsteroidal anti-inflammatory drug (NSAID), aspirin, and/or anticoagulant therapy should be stopped for 3 to 7 days before and after the procedure.Patient and caregiver education should be performed as follows:
 Explain that if the pump is not explanted at the patient’s death, the pump and alarm should be inactivated.
Explain that if the pump is not explanted at the patient’s death, the pump and alarm should be inactivated. For a single injection, the initial intrathecal injection should be 0.3 to 0.5 mg of morphine or half the intrathecal equivalent of daily opioid intake.
For a single injection, the initial intrathecal injection should be 0.3 to 0.5 mg of morphine or half the intrathecal equivalent of daily opioid intake.Procedure
Step 1: Pump Preparation
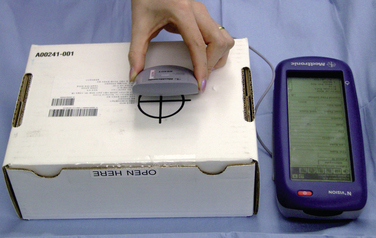
2. Check initial pump status (Fig. 16-9):
a. Use a programmer to check current pump status. Ensure that the calibration constant is the same as that shown on the package labeling.
3. Empty the pump:




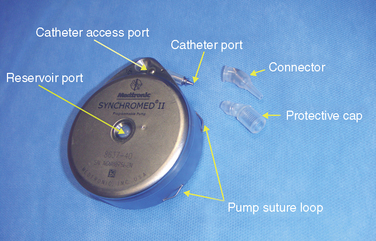
a. Remove the protective cap from the catheter port (Fig. 16-2) by pulling it away. A drop of water will appear from the catheter connector of the pump within 1 to 2 minutes. Leave the protective catheter connector cover off.
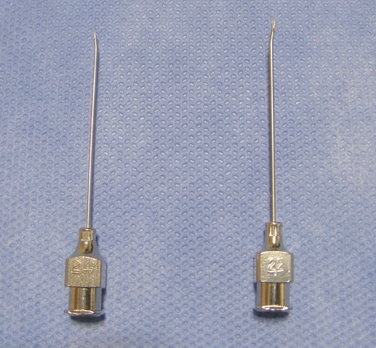
b. Insert the 22-gauge noncoring needle (Fig. 16-3) into the reservoir fill port septum until the needle touches the metal needle stop. Maintain negative pressure to withdraw the sterile water from the pump into the empty syringe. Empty the reservoir completely, until air bubbles stop flowing into the syringe (Fig. 16-11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree