Anatomy of the occipital nerve
- 1.
Vascular: irritation of the nerve by aberrant vertebral artery, dural arteriovenous fistula …
- 2.
Neurogenic: Schwanoma, C2 myelitis, multiple sclerosis
- 3.
Muscular and osteogenic: C1/C2 athrosis, atlodendal sclerosis, cervical chondroma, exuberant callus formation.
The nature of this neuralgia is not well understood. The etiology is variable with idiopathic or secondary cases: post-traumatic, compressive (C1–C2 osteoarthritis, inflammation within a context of rheumatoid polyarthritis or spondyloarthropathy, ligamento-muscular, vascular, or tumoral), postsurgical (treatment of Chiari malformation, surgery of the cervical spine with a posterior approach, surgery of the posterior fossa).
15.3 Treatments
There are a variety of treatments for occipital neuralgia, ranging from medical treatment to invasive surgery. Medical treatments include analgesics, antidepressants, anti-epileptics, topical agents, physiotherapy, osteopathy, and acupuncture. Local infiltrations of anesthetics or corticosteroids are proposed in cases refractory to medical treatment. If this fails, more invasive treatments are used, such as pulsed radiofrequency, occipital nerve neurolysis, selective rhizotomy at C1–C3 and C2 ganglionectomy [3–7].
A variety of surgical treatments do exist, but they remain imperfect in terms of pain control and may also induce side effects, such as deafferentation pain. Neuromodulation techniques offer a new approach for the treatment of occipital neuralgia. These techniques have already proved effective for migraine and cluster headache [8–15].
In 2011, Slavin [16] traced the history of peripheral nerve stimulation. Its use for the treatment of chronic pain was proposed for the first time by En 1967 by Wall and Sweet with the first implantations made in 1962 by Shelden. The author had shown that the stimulation of peripheral nerves caused a suppression of the perception of pain. Semi-experimental use of this technique continued for 15–20 years. At the end of the year 1980, peripheral nerve stimulation became a recognized surgical technique. At the end of the year 1990, Weiner and Reed proposed the use of such a percutaneous technique of inserting an electrode near the occipital nerves to treat neuralgia occipital. Weiner has shown that this percutaneous stimulation technique is simple and effective. This exploratory work marked the beginning of the modern era of peripheral nerve stimulation. Occipital nerves stimulation is proved to be efficient in occipital neuralgias and cervicogenic headache.
The physiopathologic mechanisms underlying the efficacy of occipital stimulation are still not well known. The gate control theory of Wall and Melzack could play a part in the analgesic action, but is unlikely to be the sole explanation. An involvement of the trigemino-cervical complex most likely contributes to the analgesic effect and partly explains the analgesic effect of occipital stimulation in refractory chronic headaches [17–19].
15.4 Indications for Occipital Stimulation
15.4.1 Evaluation of the Disease
Patients who suffer from refractory occipital neuralgias according to the IHS criteria will be candidate to occipital stimulation. It is a major criterion to have homogenous studies, but it is not sufficient. Occipital neuralgias must be refractory to medical treatment (association of neuropathic medication like antiepileptic, and/or antidepressant, and/or antalgic treatment like paracetamol, tramadol, or morphine) and pain management in a pain Unit, including multidisciplinary approach, physiotherapy, block test, radiofrequency rhizolysis, and/or corticosteroid infiltration of C2. An essential prerequisite to ensure an acceptable benefit/adverse effect balance is that the patient must have been informed, before any decision to operate, about the possible beneficial effects and possible complications of surgery, and must have realistic – and not unrealistic – expectations in relation to this type of treatment. ON must be chronic (duration of the disease up to 6 months). Pain must be neuropathic pain associated or not with cervicogenic headache. Pain could be unilateral or bilateral. A cranial and cervical spine MRI must be done to eliminate etiology of pain which could be relevant to a surgical treatment (tumors, aneurysms, and spine instability)
15.4.2 Preoperative Clinical and Psycho-Social Evaluation
This evaluation is essential before considering any invasive procedure for the treatment of pain, in order to select the best candidates for these techniques, to inform the patient about the potential benefits, and to limit the patient’s expectations of a miracle cure. This assessment needs to confirm the refractory nature of the neuropathic pain, to identify the presence of painful physical comorbidities (particularly an association with fibromyalgia) and/or psychological comorbidities, to record the efficacy of previous drug and non-drug treatments, and to determine the absence of general contraindication to neurostimulation procedures (coagulopathy, severe cardiac disease, chronic infections, and other comorbidities). Finally, functional surgery must not be considered if the patient has a life expectancy of less than 6 months.
A thorough psychological and/or psychiatric assessment is very important prior to any interventional technique. Ideally, the opinion of the psychologist or psychiatrist should be integrated into the multidisciplinary consultation meeting. In particular, it is important to evaluate patient’s expectations, fears, and beliefs in relation to the stimulation technique, as well as the presence of any severe or decompensate psychiatric co-morbidity (particularly severe depression or a psychotic illness); any addictive behaviors contraindicating a surgical procedure; a poor compliance and/or insufficient understanding of the treatment and a lack of social and/or family support; and any litigation, which should be settled, whenever possible, before any operative procedure and/or patients awaiting financial compensation.
15.5 Surgical Techniques
15.5.1 General Considerations
These stimulation techniques appear to be useful in various refractory neuropathic pain indications, as long as there is some preservation of sensation in the painful area. However, large-scale randomized controlled studies are necessary to confirm the maintenance of the efficacy and to validate emerging indications.
Occipital nerve stimulation (ONS, performed for the first time by Weiner and Reed [20]) is a simple technique that can be performed under local or general anesthesia. It consists of subcutaneous placement of a flat or cylindrical stimulation electrode in contact with the occipital nerves via a retromastoid or midline incision. The electrode is then connected to a stimulator placed under the skin in the infraclavicular or abdominal region.
The patient is placed in a prone position and the first stage of the procedure is performed under mild sedation or local anesthesia to monitor the patient’s feedback and ensure the most optimal coverage of the painful areas by stimulation. After disinfection and sterile draping, a curved needle is inserted at the emergence of Arnold’s nerve through midline or lateral incision. Fluoroscopic and/or echography could be used to be sure of the position of the lead with one or more contacts crossing the nerve. The electrode is sutured in place with nonabsorbable sutures using two plastic anchors. If the trial is successful, defined as more than 50% of pain reduction, the second stage (implantation of the pulse generator) is done under general anesthesia. A subcutaneous pocket is created below the clavicula, on the flank, on the buttock according to the center habits. Extension cables, if needed, are tunneled and connecting electrode and pulse generator. Otherwise, leads are directly connected to the pulse generators.
Jennifer Sweet [21] proceeds to a systematic review of occipital nerve stimulation in refractory occipital neuralgias. Nine series were analyzed: five retrospective studies, three prospective, and one unknown.
We can add our personal retrospective study performed on 60 patients with intractable occipital neuralgias treated with peripheral nerve stimulation (PNS) was performed during the period from October 2008 to October 2014. Pain evaluation, location, duration and cause, and previous treatment were analyzed. Evaluations included a visual analogue scale (VAS) before and 6 months after PNS implantation, the Medication Quantification Scale (MQS) before and 6, 12 months after implantation and at the last follow-up, failure of medical treatment and a multidisciplinary approach to pain. External trials with transcutaneous electrical nerve stimulation (TENS) were performed to evaluate if the trial is successful (patient reported at least 30% decrease of pain on VAS during 1 month). Sixty patients were implanted.
15.5.2 Paddle Lead vs. Percutaneous Lead
Paddle or percutaneous leads can be inserted via a retromastoid or a midline approach.
Retromastoid approach could be used for unilateral or bilateral approach. Several authors described a retromastoid approach to lead placement: Weiner and Reed [20], Melvin Jr et al. [22], Slavin et al. [23], Oh et al. [24], Kapural et al. [25] and Johnstone and Sundaraj [26] described implantation of paddle lead via a midline approach. Slavin used the two approaches: lateral if unilateral lead and midline if bilateral occipital neuralgias with percutaneous lead.
In our series, we use percutaneous lead and paddle lead with the two approaches like Slavin et al. [23].
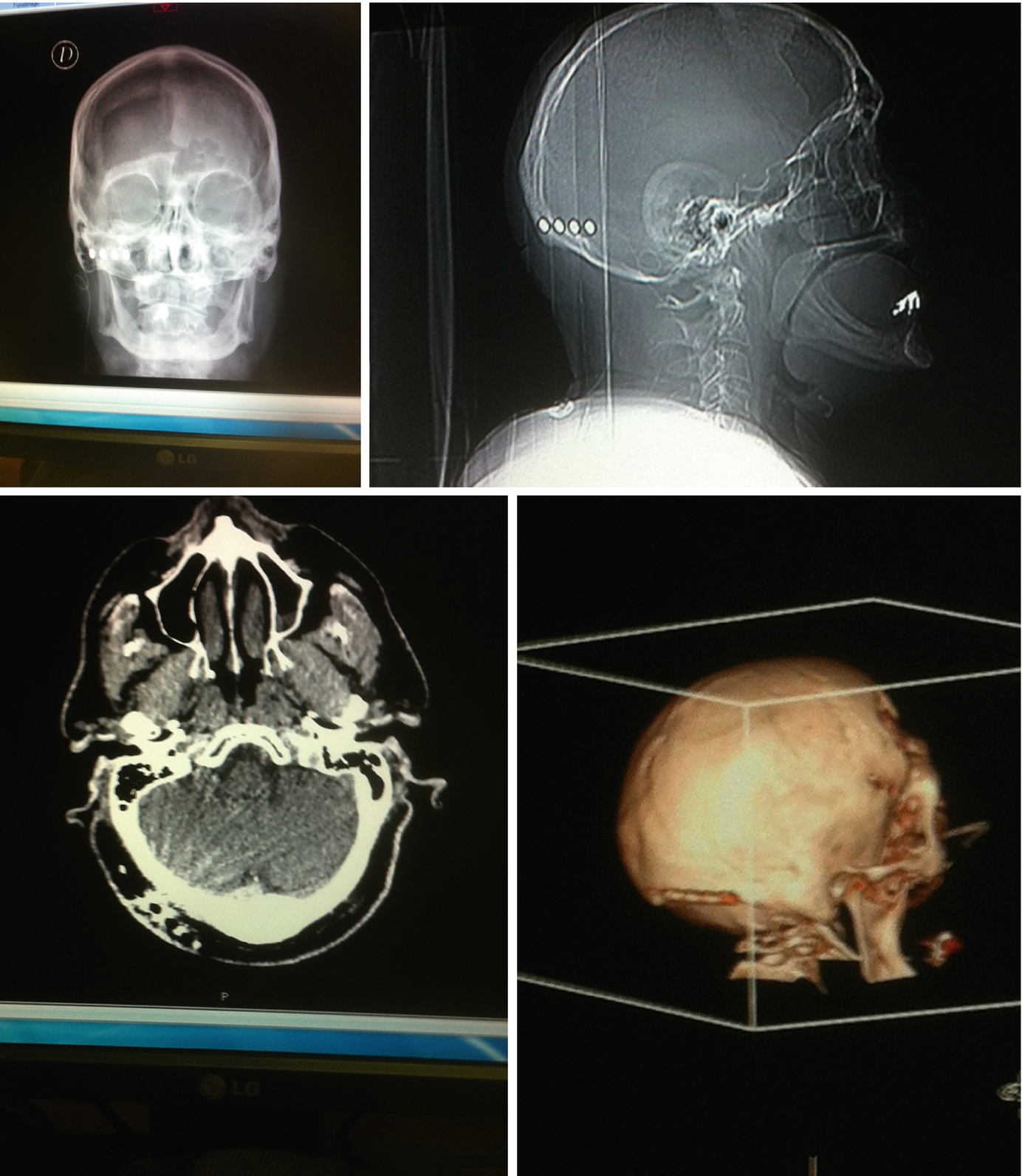
X-ray views and 3D scan views of occipital electrode
15.5.3 Local Versus General Anesthesia
This criterion is left to the appreciation of each according to local habits. If the percutaneous electrodes can be inserted under local anesthesia, it is more complicated for the surgical electrodes.
Weiner and Reed [20] described electrode placement under local anesthesia. The advantage of the local anesthesia is to be able to test the correct positioning of the paresthesia induced by the electrode covered the painful territory by the paresthesia. However, experienced physicians now perform the procedure under general anesthesia [23, 27, 28]. For a review, see the article of Paemeleire and Bartsch [19].
15.5.4 Fluoroscopy Alone or Fluoroscopy Plus Ultrasonography
- 1.
Sense of hair
- 2.
Sense of touch
- 3.
Epicritic sense
- 4.
Pressure sense
- 5.
Thermic sense
- 6.
Coarse sense
- 7.
Pain
- 8.
Protopathic sense
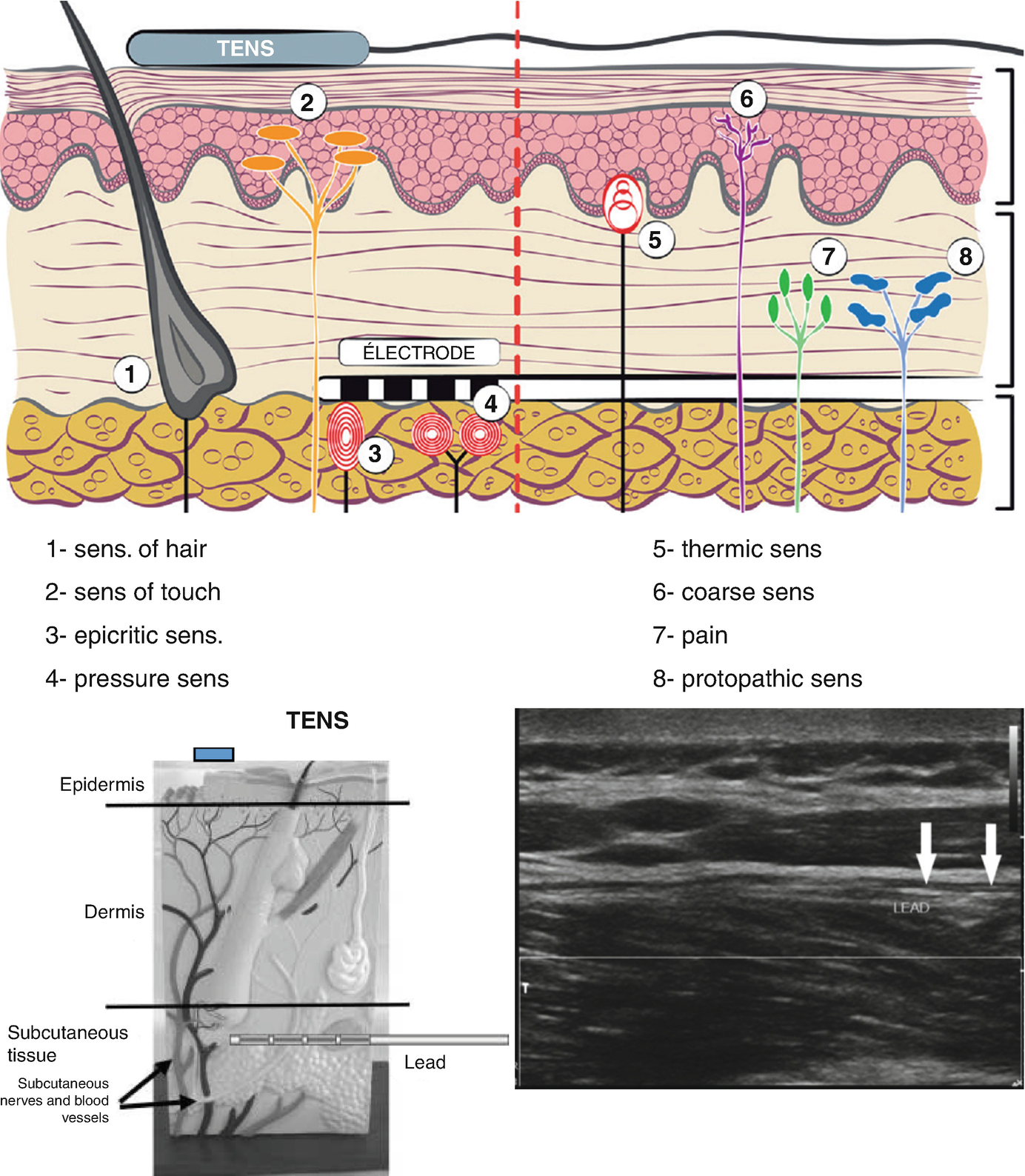
Positioning of the electrodes according to the anatomy of the sensory pathways
15.6 Results and Complications
15.6.1 Results
ONS is an effective treatment to refractory occipital neuralgias. All the articles and review provide class III-level evidence. Only three articles concern prospective study with small numbers of patients (24 patients). There is heterogeneity in evaluating the results with the use of different scales (Short-Form Mac gill questionnaire, the Visual Analog scale, the Present Pain Index, The Pain Disability Index, the percentage of pain decreased). Randomized prospective studies are needed to assess long-term results. ONS provides good results in patents with occipital neuralgia and cervicogenic headache (approximately 80% of patients experience improvement) (Weiner and Reed [20], Melvin Jr et al. [22], Slavin et al. [23], Oh et al. [24], Johnstone and Sundaraj [26], Abhinav et al. [30], Palmisani et al. [31]), and postoperative or posttraumatic occipital neuralgia (Kapural et al. [25]).
Our series is the larger series in the world. Mean Vas decreased dramatically after ONS implantation from 8.35 preoperatively to 2.32 postoperatively. The case series of Abhinav is particularly impressive with patient totally pain free. The results seem to be sustained in the long term. The study of Weiner and Reed had the more important follow-up with a mean of 2 years, ranging from 1.5 to 5.5 years.
It is difficult to conclude whether the surgical electrodes provide a greater benefit in terms of pain reduction, but there seems to be a slight superiority of these. In our series, we use less current with paddle lead and we have no migration with paddle lead.
15.6.2 Failed Trials
Some patients were not implanted because of failed trial: Melvin 3/14, Slavin 4/14, Johnstone 1/8. We don’t have this problem because we use TENS to preselect the patients [32].
We shaved the patient or use Arnold kit for the TENS. All patients who have more than 30% reduction of pain with TENS were implanted with ONS in one stage (lead and pulse generator at the same time). Patients with negative TENS had a trial with a percutaneous lead to test if there is pain reduction about 50% or more. If the trial is negative, patients do not receive the implanted pulse generator.
15.6.3 Parameters Settings
In the literature, Oh and Whiting [33] described high-intensity parameters: 8 V, 330 μs, 85 Hz at the beginning, which decreased at 2.5 V, 300 μs, 85 Hz with a cycling mode.
For Slavin et al. [23], pulse width ranged from 90 to 360 μs, the frequency ranged 30 to 90 Hz, and the amplitude ranged from 1 to 4 V.
In the study of Rodrigo-Royo et al. [34], in four cases of cervicogenic headache, pulse width ranged from 210 to 450 μs, the frequency ranged 40 to 60 Hz, and the amplitude ranged from 0.3 to 2.7 V.
The implanted material in our series was obtained from three manufacturers (Medtronic, Boston Scientific, St Jude Medical) and was distributed as follows: three Boston Scientific, five Medtronic, 51 St Jude Medical. Seventeen patients received a rechargeable stimulator.
For St Jude Medical: 4.25 mA, 50 Hz, 208 μs, the frequency varied from 30 to 80 Hz, PW ranged from 156 to 256 μs, and current intensity varied from 1.1 to 13.3 mA.
For Boston: intensity was 4.7 mA for each side, 50 Hz, 450 μs (1.2 to 17 mA).
For Medtronic: mean intensity was 3.4 V, 50 Hz, 210 μs (with intensity varying from 1.2 to 7 V and a current pulse of between 150 and 450 μs).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


