aConventional tablets.
bConcentration dependent.
cValues refer to pharmacologically active metabolite eslicarbazepine/10-hydroxycarbazepine.
dEnteric-coated tablets.
NE, not established.
The Individual Therapeutic Concentrations
Despite the shortcomings of present reference ranges, TDM may be useful by employing the concept of “individual therapeutic concentrations” using intraindividual comparisons of drug serum concentrations (3,5). The drug concentration at which a patient has an optimal effect will serve as the individual reference for comparison if future treatment failures should occur and to check if the change in the clinical status of the patient is related to pharmacokinetic alterations or not. The concept of the individual therapeutic concentration may therefore be helpful even without a well-defined reference range. The basis for TDM will rely on a relationship between drug serum concentration and clinical effect in the individual patient, depending on the mechanism of drug action and possible influence by pharmacologically active metabolites and pharmacokinetic intraindividual variability. Thus, an optimal AED treatment can be best guided by identifying the “individual therapeutic concentration” (3,5).
IMPACT OF TDM ON CLINICAL OUTCOME
Although studies of the impact of TDM on treatment outcome in terms of optimum seizure control are scarce (12–15), two randomized studies comparing treatment outcome with or without the use of TDM have been reported (14,15). Neither study, however, provided evidence for the usefulness of routine monitoring of AEDs in the general epilepsy population, which, however, does not argue against the value of TDM in special situations and clinical indications.
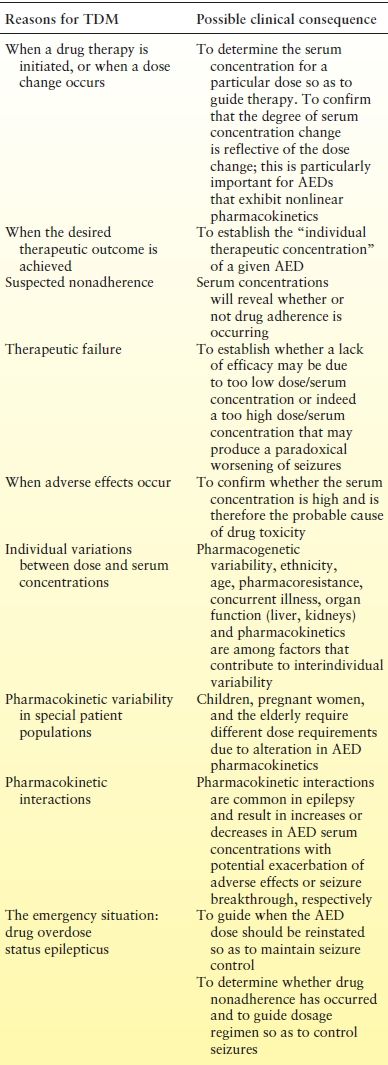
CLINICAL INDICATIONS FOR MONITORING ANTIEPILEPTIC DRUGS
Traditionally, TDM has been applied to the older first-generation AEDs (carbamazepine, phenytoin, phenobarbital primidone, and valproic acid), primarily because not only do they exhibit substantial interpatient pharmacokinetic variability but also they have narrow therapeutic indices. Nevertheless, TDM is increasingly being used to individualize treatment with the various new second- (vigabatrin, lamotrigine, gabapentin, pregabalin, levetiracetam, oxcarbazepine, tiagabine, topiramate, and zonisamide) and third-generation (lacosamide, retigabine, eslicarbazepine acetate, and perampanel) AEDs and also for the orphan AEDs (stiripentol and rufinamide), and the indications for monitoring are exactly the same for all AEDs (Table 48.2) (3,4,16). Some key pharmacokinetic characteristics that impact on TDM and the reference ranges for the various AEDs are shown in Table 48.1. Indications for AED TDM are discussed in the following sections.
Table 48.2 Clinical Indications for Monitoring Antiepileptic Drugs
Drug Interactions
Pharmacokinetic interactions with AEDs represent a major indication for AED TDM. These interactions involve changes in serum concentrations consequent to changes in metabolism, principally due to enzyme induction or inhibition and are well described (17–22). Enzyme induction can result in a decrease in serum concentrations and potentially seizure breakthrough while enzyme inhibition can result in increased serum concentrations and potentially neurologic toxicity. Typically, enzyme induction completes within 14 to 21 days, and thus TDM should be undertaken after this period has elapsed. The maximum consequence of enzyme inhibition interactions, whereby new steady-state serum concentrations are achieved, is directly dependent on the half-life of the inhibited drug and typically occurs 4–6 half-lives after the inhibiting drug is coprescribed. Thus, TDM can be readily used to characterize the time course and magnitude of these interactions and make dosage adjustments as necessary.
For most patients, seizure freedom is readily achieved by use of AED monotherapy. However, 30% of patients require polytherapy AEDs (the use of two or more AEDs), and it is these patients that are susceptible to pharmacokinetic AED versus AED interactions. Being aware of which AEDs have a propensity to cause pharmacokinetic interactions and to choose those that do not interact or have minimal propensity to interact, along with knowledge of the mechanism of interaction, is important (22). Thus carbamazepine, phenytoin, phenobarbital, and primidone are potent enzyme inducers, while valproate is a potent enzyme inhibitor, and they may affect the serum concentration of other AEDs (17). In contrast, gabapentin, levetiracetam, lacosamide, pregabalin, and vigabatrin are not likely to be involved in pharmacokinetic interactions due to the fact that they do not undergo metabolism via cytochrome P450 (CYP) or uridine glucuronyl transferase (UGT)-mediated metabolism and instead are excreted renally.
Interactions between AEDs and other drugs used to treat concurrent pathologies are also important and similarly require TDM so as to individualize treatment. These include analgesics, antimicrobials, antineoplastic agents, cardioactive drugs, immunosuppressants, psychotropic drugs, and steroids, including oral contraceptives (18,21,23).
Regarding oral contraceptives, the estrogen component enhances metabolism via UGTs so that AEDs (lamotrigine, oxcarbazepine, and valproate) whose metabolism is via this enzyme system experience decreases in serum concentrations, in some patients by up to 50% (24,25). Conversely, many AEDs (carbamazepine, phenytoin, and phenobarbital) enhance the metabolism of oral contraceptives and compromise contraceptive control.
It should be remembered that when an interacting drug is withdrawn, the interaction will go in reverse, and therefore it is important to reverse any dosage adjustments made accordingly and is best guided by TDM. Finally, identification of pharmacodynamic interactions can similarly benefit from TDM because any change in the clinical status of a patient (seizure exacerbation or increased adverse effects) that is not associated with a change in serum AED concentration is concluded by default as a pharmacodynamic interaction.
Special Patient Populations
Children and Adolescents
Children differ from the adult population due to rapid changes in pharmacokinetics during infancy and early childhood, due to physiologic alterations early in life. For most AEDs studied in youngest children, the elimination half-life is reduced because the clearance is high, especially from 6 months of age and to about 6 years of age. The volume of distribution may also differ (2,4,26). The clinical consequence is that children will need a higher dosage per kilogram of body weight than in older children or adults. This pharmacokinetic variability in children makes it difficult to predict optimal doses and serum concentrations, and thus, TDM is particularly helpful in these patients.
Pregnant Women
In pregnant women, rapid changes in pharmacokinetics are expected as a consequence of various physiologic changes including decreased absorption, altered distribution, decreased protein binding, increased metabolism due to increased enzyme capacity in the liver CYP isoenzymes and UGTs, and increased drug excretion (4,27,28). During this period, TDM is particularly helpful in patient management. For highly protein-bound drugs, management may best be guided by measurement of unbound concentrations (29). Significant pharmacokinetic changes have been recorded for lamotrigine, with an increase in clearance of 50% to 330%, with the most pronounced effect occurring in late pregnancy (28–32). Levetiracetam plasma concentrations can decrease by 60% in the third trimester compared to baseline (33). The metabolism of oxcarbazepine also increases during pregnancy due to enhanced glucuronidation, as the concentrations of the most active enantiomer (S-(+)-10-hydroxycarbazepine) metabolite is increased 1.5- to 13-fold after delivery. Serum concentrations of topiramate also appear to decline gradually throughout pregnancy. The mechanisms are not known, but increased glomerular filtration may play a major role (33,34). Other AEDs that are associated with decreases in serum concentrations during pregnancy and that may benefit from TDM so as to optimize therapy include felbamate, gabapentin, pregabalin, tiagabine, vigabatrin, and zonisamide (28,35,36).
Elderly
The oral clearance of almost all AEDs is reduced in the elderly. Reductions of 10% to 90% have been observed but are most often in the range of 30% to 50% (2). The elderly, with increased morbidity, exhibit pronounced physiologic alterations that affect the pharmacokinetic characteristics of AEDs namely decreased absorption, altered distribution, decreased capacity of metabolizing enzymes, decreased blood flow to eliminating organs, and impaired hepatic and renal function. Additionally, age-related changes in pharmacodynamics and an increased likelihood of drug interactions, because of treatment of concomitant comorbidities, affect the efficacy and safety not only of AEDs but also of concomitant therapy. TDM can guide attainment of targeted concentrations and maintaining these concentrations over time, especially as comedications are added or discontinued. For highly protein-bound AEDs, measurement of unbound concentrations may be indicated. Interpretation of drug concentrations in the elderly should also take into account the fact that the elderly may show increased pharmacodynamic sensitivity to AEDs, and therefore, therapeutic and toxic effects may develop at relatively low concentrations. Thus, interpretation of drug concentration measurements should be interpreted more cautiously.
Hepatic and Renal Disease
Most AEDs are metabolized by the liver, and thus, hepatic disease may alter their clearance (4). In addition, as the liver is the source of many proteins, serum protein binding may also be affected. Studies evaluating serum AED concentrations during hepatic illness are sparse, and there is no test that will predict the degree of change in clearance. Thus, in any person with hepatic disease, AED TDM should be undertaken, and for highly bound AEDs, free concentration monitoring may better guide treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








