Fig. 1
This figure shows occurrence view of STRING analysis for queried genes among different mammals. The following gene viz. Orx, OX1R, OX2R, ADRA1, ADRA2, ADRB1, TH, and DBH, known to be responsible for synthesis of Orx and NA as well as their receptors were submitted for analysis. No homolog of Orx-gene was detected in Dasypus noveminctus and Ornithorhyncus anatinus. Abbreviations α1 adrenoceptor—ADRA1, α2 adrenoceptor—ADRA2, β adrenoceptor—ADRB1, tyrosine hydroxylase—TH, and dopamine β hydroxylase—DBH
2.2 Support from Clinical Studies (Narcolepsy)
Narcolepsy is characterized by disorganized sleep-wakefulness cycle and cataplexy (Tsujino and Sakurai 2009; Weinhold et al. 2014) and increased REMS (Chemelli et al. 1999; Willie et al. 2003). Loss of Orx has been considered to be among the primary causes of narcolepsy (Kilduff and Peyron 2000; Sutcliffe and de Lecea 2002). It has been shown that ~90 % of Orx-ergic neurons are lost in human narcolepsy with cataplexy (Burgess and Scammell 2012). Lack of Orx signalling contributes to narcolepsy was further confirmed by the findings from post-mortem brains of human narcolepsy-cataplexy patients that showed a dramatic loss of Orx-mRNA and Orx-immunoreactive neurons (Peyron et al. 2000; Thannickal et al. 2000). Orx-A level is undetectable in cerebro-spinal fluid of most human narcoleptic patients (Nishino et al. 2009). Many of the narcolepsy symptoms, e.g., rapid state-transitions or inability to remain awake for long and sudden episodes of cataplexy during waking behavior are also exhibited by Orx-receptor knockout mice and Orx/ataxin-3 transgenic mice and rats with ablated Orx neurons (Siegel 2004; Ohno and Sakurai 2008). Orx deficiency in humans and animals produces characteristic symptoms of REMS disorder and narcolepsy (Peyron et al. 1998; Chemelli et al. 1999). These findings provide the basis to conclude the Orx is directly or indirectly involved in REMS regulation. Also, the findings suggest that Orx normally facilitates alertness and muscle tone and inhibits REMS and associated atonia.
2.3 Support from Experimental, Anatomical and Physiological Studies
Several lines of evidence support the involvement of Orx neurons in the regulation of sleep-waking-REMS; however, their detail mechanism of action is not clearly understood yet. Administration of Orx (icv) in rats increased arousal (Thakkar et al. 1999; Bourgin et al. 2000). Chemical (Thakkar et al. 1999; Alam and Mallick 2008), electrical (Choudhary et al. 2014) or optogenetic (Carter and de Lecea 2011; Carter et al. 2012, 2013) stimulation of PeF-Orx-ergic neurons induced arousal and reduced REMS. An accepted molecular correlate of neuronal activity, the c-fos expression, increased in PeF-Orx-ergic neurons during extended waking as compared with normal sleep-waking (Estabrooke et al. 2001). REMS duration as well as frequency was significantly increased in Orx-knockout mice (Chemelli et al. 1999; Willie et al. 2003). Orx-A injection into the anterior hypothalamus (Methippara et al. 2000), LC (Bourgin et al. 2000; Smith et al. 2003) and LDT (Bernard et al. 2003, 2006; Nunez et al. 2006) modulated REMS. Also, reduced level of Orx and reduced number of Orx-ergic neurons have been reported in animals showing increased REMS (Chemelli et al. 1999; Nishino et al. 2000; Gerashchenko et al. 2001; Hara et al. 2001; Beuckmann et al. 2004). Orx neurons showed increased activity during waking and are quiescent during NREMS as well as during tonic phase of REMS (Lee et al. 2005; Mileykovskiy et al. 2005). In this context it is expected that Orx level is likely to decrease during REMS and if these neurons are kept active, REMS and NREMS should be prevented and waking should increase. As a corollary, it is also expected that Orx level should increase during increased physical activity and REMS loss. Indeed it has been show that long term activation of the PeF-neurons reduced REMS (Choudhary et al. 2014) and Orx level increased in the LC after REMS deprivation (REMSD) (Mehta et al. 2015).
2.4 Relationship of Orx-ergic Neurons with REMS Regulating Areas
The Orx neurons in the PeF are the primary source of Orx in the brain and project to all parts of the brain, with moderate to dense innervations to LC, the raphe, the tubero-mammillary nucleus (TMN), and the LDT/PPT (Peyron et al. 1998; Greco and Shiromani 2001; Marcus et al. 2001; Gerashchenko et al. 2003); the LC probably receives the maximum inputs (Horvath et al. 1999; Kilduff and Peyron 2000; Sutcliffe and de Lecea 2000). Orx-ergic neurons also receive extensive inputs from the LC, ventral tegmental area (dopaminergic neurons), raphe, TMN; pontine reticular formation; LDT/PPT and the basal forebrain (Yamanaka et al. 2003, 2006; Muraki et al. 2004; Yamanaka 2006).
Normally REMS does not appear during waking and it appears only after a period of NREMS has set in. Thus, REMS may be modulated by direct input on REMS regulatory apparatus, or indirectly by modulating the waking and NREMS regulatory apparatus. Based on several studies we have concluded that cessation of LC-REM-OFF neurons is a necessity for the generation of REMS (Pal and Mallick 2007). Further, simulation studies have shown that REMS does not appear if the waking inputs on the REMS active neurons is not withdrawn (Kumar et al. 2012). Several lines of evidence so far suggest that Orx has profound influence on the muscle activity so that the latter remains active. This helps the animal to keep oneself active (physically as well as mentally/cognitively) so that they can hunt and gather food for survival (Mieda and Yanagisawa 2002; Mieda et al. 2006). This view may be supported by the fact that role of Orx in feeding and hunger (Burdakov et al. 2005; Clark et al. 2009) has also been established. In line with this hypothesis, we proposed and confirmed as explained below that the Orx influences REMS by modulating the LC-neurons, the REMS regulatory apparatus and thereby secondarily influences REMS.
3 Role of NA-LC Neurons in REMS
The neurons in the LC, the major source of NA in the brain, are active during wakefulness, less active during NREMS, and quiescent during REMS (Hobson et al. 1975; Aston-Jones and Bloom 1981) and their cessation is a pre-requisite for REMS regulation (Mallick et al. 2012). Electrical or pharmacological activation of LC neurons reduced REMS (Singh and Mallick 1996; Kaur et al. 2004). NA level is reduced during REMS (Berridge and Abercrombie 1999; Shouse et al. 2000), while it is elevated during REMSD (Porkka-Heiskanen et al. 1995). In a recent study using optogenetic stimulation it has been confirmed that activation of the LC-NA-ergic neurons reduced REMS and increased waking (Carter et al. 2010).
4 Evidences Favouring Interactions Between Orx-Ergic and NA-Ergic Neurons for REMS Regulation
(i) There are to and fro connections between the PeF-Orx-ergic and NA-ergic LC neurons (Baldo et al. 2003; Yamanaka et al. 2003; Espana et al. 2005; Li and van den Pol 2005; Yamanaka 2006); (ii) Orx depolarizes LC neurons (Ivanov and Aston-Jones 2000) and microinjection of Orx into the LC increases wakefulness and reduces NREMS and REMS (Bourgin et al. 2000). Similarly, Orexin-1 receptor (OX1R) knockout in LC increases REMS in mice (Chen et al. 2010); (iii) electrical or chemical stimulation of Orx-ergic neurons modulates waking, NREMS and REMS (Alam and Mallick 2008; Choudhary et al. 2014; Kostin et al. 2014); (iv) NA regulates Orx neuronal activity (Bayer et al. 2005); (v) in vivo studies demonstrated that administration of Orx-A but not Orx-B agonist into the LC of rats suppressed REMS and increased wakefulness at the expense of REMS and NREMS (Bourgin et al. 2000); (vi) excitatory effect of Orx-A on LC neurons in vivo have been demonstrated by increased firing rate in single unit recording from LC after iontophoretic application of Orx-A (Bourgin et al. 2000); (vii) the Orx acting through OX1R modulates NA release (somato-dendritic as well as axon terminal) from LC neurons (Chen et al. 2008) in a dose dependent manner; (viii) the observation in (vii) above may be supported by the findings from in situ hybridization study that the OX1R is exclusively expressed in NA-ergic LC neurons (Mieda et al. 2011); (viii) further, in narcolepsy although the patients can express muscle tone, upon exposure to specific excitement causing factor, there is reduced muscle tone (cataplexy) and there is increased REMS (or like state).
We know that as long as the LC neurons are kept active the REMS does not appear (Mallick et al. 2012) and that the LC neurons are kept active by the wake active brain areas (Thankachan et al. 2001) as well as by Orx (Hagan et al. 1999; Horvath et al. 1999). Therefore, it is likely that Orx-ergic neurons are one of the many components for keeping the LC-neurons active that keeps REMS and its associated muscle atonia at bay during waking. We propose that in normal condition these Orx-ergic neurons possibly are activated by several psychic (excitement) inputs, which keep the LC-neurons active. However, in narcolepsy those excitement related inputs are lost (withdrawn) because the Orx-ergic neurons are known to be lost or reduced in number in narcolepsy (Kilduff and Peyron 2000; Sutcliffe and de Lecea 2002); also we suppose there is likely to be genetic predisposition, which needs to be confirmed. The possible cause of loss of Orx-ergic neurons has been discussed below. So far direct evidence in support of central regulation of Orx-induced muscle tone increase is lacking. However, Orx-ergic activation of LC neurons and muscle tone activation has been reported (Kiyashchenko et al. 2001). NA-ergic systems have been shown to maintain the muscle tone during arousal and this regulation is possibly lost in narcoleptic canines and thus, cataplexy is expressed (Monti et al. 1988; Crochet and Sakai 1999; Wu et al. 1999; Shouse et al. 2000; Kiyashchenko et al. 2001). Also, behavioral instability was observed in NA-deficient models (Monti et al. 1988; Delagrange et al. 1993). Finally, in a recent study in rats we have observed that after REMSD Orx-A level increases in the LC but not in the PPT (Mehta et al. 2015) support our contention.
5 Orexinergic Effects Are Mediated Through the LC-Neurons
So far we discussed the indirect evidence supporting Orx-ergic influence on muscle atonia (and possibly REMS) is mediated through the LC neurons. As direct evidence in a recent in vivo study in rats, we have confirmed the same. In brief, chronic rats were surgically prepared with two pairs of cannulae implanted bilaterally into the PeF and the LC. The rats also had electrodes for recording electrophysiological signals for identification sleep-waking-REMS. In these surgically prepared chronic rats, stimulation of PeF neurons by glutamate microinjection reduced REMS and increased waking. However, the following day in the same rats the effects of PeF stimulation on REMS were prevented if Orx-ergic receptor antagonist (SB-408124) was simultaneously injected into the LC bilaterally (Choudhary et al. 2014) the scheme of the experimentation has been shown in Fig. 2. The finding of this study confirmed that the effect of stimulation of PeF-Orx-ergic neurons on REMS is mediated primarily by activating the LC neurons. Our recent finding that Orx-level in the LC increases after REMSD (Mehta et al. 2015) also support our contention.
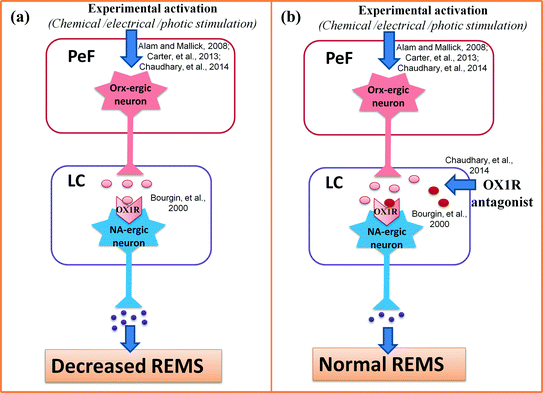

Fig. 2
Neural connection from PeF to LC for modulation of wakefulness and REMS has been illustrated. Left panel (a) depicts that chemical, electrical or photic stimulation of the PeF neurons have been reported to increase wakefulness and decrease REMS; while the right panel (b) shows that reduced REMS as in a was prevented by simultaneous microinjection of OX1R antagonist into the LC. Abbreviations as in the text
6 Justification in Support of Orx-Induced Effects Are Mediated by NA Released from LC Neurons—Validity of Our Contention
So far we have discussed that increased activity of the Orx-ergic neurons reduces REMS by activating the LC neurons. This suggests that increased Orx-induced loss of REMS-associated pathological effects should be mediated by NA released from the LC neurons and reduction of Orx-ergic neurons should increase REMS. We would now attempt to explain some of the processes in support and to validate our contention. It has been reported that increased activity of Orx-ergic neurons is associated with reduced REMS (Alam et al. 2002) and loss of Orx-ergic neurons is associated with narcolepsy, when the REMS is increased (Chemelli et al. 1999; Nishino et al. 2000; Gerashchenko et al. 2001; Hara et al. 2001; Beuckmann et al. 2004). Although the detailed mechanism is unknown, our following explanation-cum-proposition provides a testable hypothesis (model). The increased activity of the LC neurons has been reported to induce waking and prevent occurrence of REMS i.e. reduced REMS (Singh and Mallick 1996; Carter et al. 2010). The LC neurons receive inputs from the wake promoting areas and PeF is one such area (Espana et al. 2005). Further, to and fro connections between the PeF and LC neurons have been reported (Baldo et al. 2003; Yamanaka et al. 2003; Espana et al. 2005; Yamanaka 2006). If the balance between the PeF-LC-PeF neuronal input/output is disturbed so that NA level in the PeF is elevated, it would induce PeF neuronal loss. Reduced number of PeF neurons would withdraw the excitatory PeF input from the LC neurons and it would be at least one reason for increased REMS, because LC neuronal activity is necessary to prevent appearance of REMS (Pal and Mallick 2007; Mallick et al. 2012). This model may be supported by the fact that REMSD induced elevated NA has been reported to cause increased neuronal apoptosis (Majumdar and Mallick 2005; Biswas et al. 2006; Ranjan et al. 2010) and loss of PeF neurons associated with increased REMS has been reported in narcolepsy (Chemelli et al. 1999; Nishino et al. 2000; Gerashchenko et al. 2001; Beuckmann et al. 2004). Finally, based on the findings and discussions we have summarized in Fig. 3 the possible neural mechanism for PeF-induced LC-mediated effect on REMS and its loss-associated patho-physiological changes.
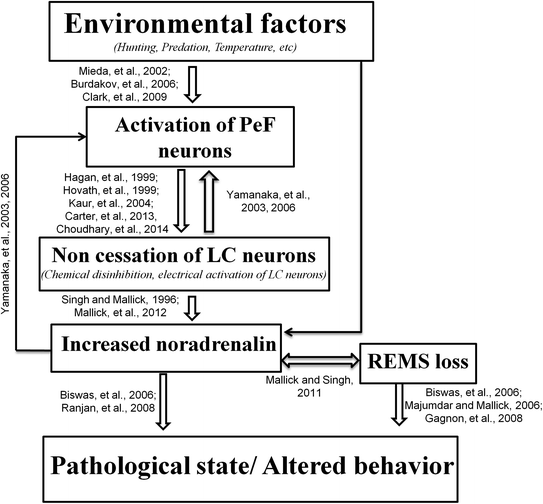

Fig. 3
PeF-induced LC-mediated modulation of REMS and its loss-associated patho-physiological changes have been schematically represented in this figure. Abbreviations as in the text
7 Conclusion
Orx-ergic neurons in the PeF are wake active, there are PeF-LC-PeF connections and Orx stimulates LC neurons. The PeF neurons modulate the LC neurons possibly to keep the animals active in doing normal activities. However, if the input/output of the PeF-LC-PeF is disturbed so as to elevate NA in the PeF, the latter neurons are lost, causing withdrawal of excitatory input from the LC-REM-OFF neurons. The latter is responsible for muscle atonia and increased REMS, the classical symptoms of narcolepsy.
Acknowledgments
Research funding from Indian funding agencies viz. CSIR, DBT, BUILDER support, DST, J.C. Bose fellowship and UGC Resource Networking program are acknowledged.
References
Alam MA, Mallick BN (2008) Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci Lett 439:281–286PubMed
Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R (2002) Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol 538:619–631PubMedCentralPubMed
Allada R, Siegel JM (2008) Unearthing the phylogenetic roots of sleep. Curr Biol 18:R670–R679PubMedCentralPubMed
Aristakesian EA (2009) Evolutionary aspects of sleep and stress interaction: phylo-, ontogenetic approach. Zh Evol Biokhim Fiziol 45:598–611PubMed
Aserinsky E, Kleitman N (1953) Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118:273–274PubMed
Aston-Jones G, Bloom FE (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1:876–886PubMed
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







