Infection and Inflammatory Diseases
Oscar H. Del Brutto
Introduction
Infectious and inflammatory diseases of the central nervous system (CNS) cause a wide range of clinical manifestations, including decreased level of consciousness, behavioral changes, increased intracranial pressure, focal neurologic deficits, and seizures. The latter may occur as the primary manifestation of the disease or as part of a diffuse encephalopathy. Pathogenesis of the seizure disorder varies widely from one disease to another. In some of these conditions, seizures appear in close temporal association with the acute disease process, although in others, seizures may occur from several weeks to months later, and tend to recur over the following years. Recognition of infectious- or inflammatory-related acute or remote symptomatic seizures has important therapeutic and prognostic implications. Here, we review the most common CNS infectious and inflammatory disorders associated with seizures.
Parasitic Infections
Neurocysticercosis
Neurocysticercosis (NCC) is defined as the infection of the CNS by the larval stage of Taenia solium. The disease occurs when humans become intermediate hosts in the life cycle of this cestode after ingesting its eggs in contaminated food or by the fecal–oral route in individuals harboring the adult parasite in the intestine. Although the former was previously considered the most common form of transmission, recent studies showing clustering of NCC patients around taeniasic individuals have changed previous concepts crediting food and the environment as the main sources of human contamination with T. solium eggs.21 Taenia carriers are contagious sources of cysticercosis, endangering everyone coming into contact with them. Human cysticercosis must be considered a disease resulting from contagion from an infected human; therefore, the patient’s close environment should be investigated to eradicate the source of contagion.
NCC constitutes a threat to millions of people in Latin America, sub-Saharan Africa, and Asia. Massive immigration of people from endemic areas has also caused a recent increase in the prevalence of this parasitic disease in the United States and some European countries.68 NCC is a leading cause of late-onset epilepsy and a major cause of seizures in developing countries, where the prevalence of active epilepsy is twice that seen in industrialized nations. Indeed, population-based studies have shown that NCC accounts for up to 30% of this excess fraction of epilepsy in the developing world.14,39,41 It is estimated that 50,000 deaths due to NCC occur every year, and many times that number of patients survive but are left with irreversible brain damage. Despite the magnitude of these numbers, they are but the “tip of the iceberg” because the actual prevalence of NCC is not known.
Pathophysiology
Cysticerci may be located in brain parenchyma, subarachnoid space, ventricular system, and spinal cord. After entering the CNS, cysticerci elicit few inflammatory changes in the surrounding tissues. In many patients, cysticerci remain in this vesicular stage for years. In others, parasites enter, as the result of the host’s immune attack, in a process of degeneration. Stages of involution through which cysticerci pass during this process are called colloidal, granular, and calcified. Inflammatory reaction around cysticerci induce pathological changes in the CNS serving as a substratum for the further development of seizures. Within the brain parenchyma, such a reaction is usually associated with edema and reactive gliosis. At the subarachnoid space level there is thickening of the leptomeninges with entrapment of cranial nerves and blood vessels located at the base of the skull. Luschka and Magendie’s foramina may be occluded with the subsequent development of hydrocephalus. Ventricular cysticerci elicit a local inflammatory reaction if they are attached to the ventricular wall. In such cases, ependymal cells proliferate and may block cerebrospinal fluid (CSF) transit at the level of the cerebral aqueduct or Monro’s foramina; this process of granular ependymitis causes obstructive hydrocephalus.49
Epilepsy is more frequently observed in patients with cysticerci located in the brain parenchyma or in the depth of cortical sulci. Cysticerci in all stages of involution may induce seizures, although the mechanisms of epileptogenesis are different. Vesicular cysts cause seizures due to compression of the surrounding brain parenchyma and colloidal cysts cause seizures due to acute inflammatory changes. In contrast, granular and calcified cysticerci cause seizures due to the intense astrocytic gliosis that usually surrounds these lesions.44
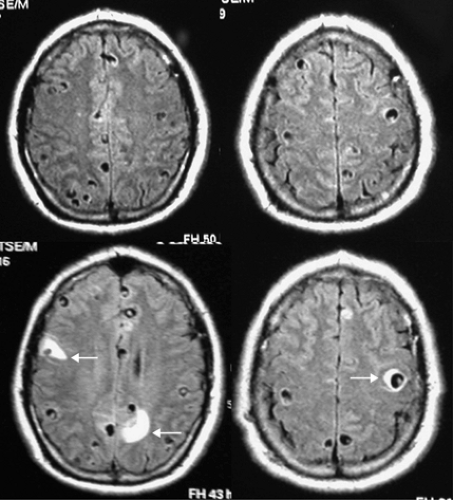
The concept that cysticerci play a role in epileptogenesis comes from epidemiological studies showing a correlation between populations with increased prevalence of both cysticercosis and epilepsy, and from neuroimaging studies showing NCC in patients with epilepsy in the absence of other etiologies. Also, the episodic appearance of edema surrounding cysticerci after a seizure episode unquestionably links cysticerci with seizures (Fig. 1). This phenomenon occurs not only in patients with living cysts but also in those with calcifications, suggesting that calcified cysticerci should not be seen as inert lesions causing no symptoms.
The pathogenesis of edema around calcified cysticerci is not fully understood.44 One hypothesis is that periodic remodeling of calcifications exposes the host’s immune system to residual antigens still present in these lesions. Direct calcium toxicity is another possibility as it has been suggested that brain lesions that calcify are associated with increased seizure activity compared to those that fail to calcify. It is also possible that brain gliosis left during the death of the parasite explain recurrent
seizure activity. Another hypothesis is that edema is not the cause but the result of the seizure, as has been documented in a few patients after a cryptogenic partial status epilepticus. However, this is improbable because the magnetic resonance imaging (MRI) pattern of perilesional edema in cysticercosis is most consistent with vasogenic edema resulting from breakdown of the blood–brain barrier, and does not have the imaging appearance of cytotoxic edema resulting from cell swelling associated with prolonged seizures.
seizure activity. Another hypothesis is that edema is not the cause but the result of the seizure, as has been documented in a few patients after a cryptogenic partial status epilepticus. However, this is improbable because the magnetic resonance imaging (MRI) pattern of perilesional edema in cysticercosis is most consistent with vasogenic edema resulting from breakdown of the blood–brain barrier, and does not have the imaging appearance of cytotoxic edema resulting from cell swelling associated with prolonged seizures.
Clinical Manifestations
NCC is highly pleomorphic owing to individual differences in the number and location of the lesions. Seizures, focal deficits, cognitive decline, and increased intracranial pressure are common manifestations of NCC. Seizures occur in more than 70% of NCC patients.15 Most of these patients have a normal neurologic examination, and differ from patients with epilepsy due to other cerebral lesions, who usually present with focal signs.
Seizures due to NCC are most commonly simple partial or generalized tonic-clonic, although some patients may present with complex partial or myoclonic seizures. The seizure type has been considered to be related to the number and location of the parasites, whereby patients with a single lesion present with partial seizures, although patients with multiple lesions have generalized seizures.15 However, other studies have shown no difference in the frequency of partial seizures in patients with single cysts as compared with those with multiple cysts.40 It is possible that most NCC patients with generalized seizures actually have partial seizures with rapid secondary generalization, an assumption based on the fact that focal brain lesions rarely course with genuine generalized seizures. Not all NCC patients with seizures develop epilepsy. Indeed, there are some patients with a single colloidal cyst who after a bout of two or three seizures remain free of seizures even without antiepileptic drug (AED) therapy.53 Nevertheless, if we consider the population of patients with NCC-related seizures at large, the vast majority actually have epilepsy because the epileptogenic focus is already developed when the patient is first seen.
Diagnosis
NCC is often diagnosed on the basis of information provided by neuroimaging studies and serology. Both computed tomography (CT) and MRI give objective information on the number and location of lesions as well as on the stage of evolution of cysticerci.19 Although MRI has better accuracy than CT, it may miss some small calcifications and has the shortcoming of being less available in endemic areas for NCC. From the many immune diagnostic tests, current evidence favors the use of serum immunoblot using purified glycoprotein antigens.21 A major problem using the immunoblot is that almost 50% of patients with a single intracranial cyst may test negative, and that some patients with taeniasis (but not NCC) may test positive. A set of diagnostic criteria has been proposed to homogenize the diagnosis of NCC.13 Proper interpretation of these criteria permit two degrees of diagnostic certainty, definitive or probable (Table 1).
Table 1 Diagnostic criteria for neurocysticercosis | |
|---|---|
|
Treatment
Characterization of NCC according to the viability and location of lesions is important for a rational therapy, which usually includes a combination of symptomatic and cysticidal drugs, surgical resection of lesions, or placement of ventricular shunts.20 Albendazole and praziquantel have changed the prognosis of most patients with parenchymal brain and
subarachnoid cysts presenting with seizures. However, because pioneer studies of NCC therapy focused on the number of cysts before and after the trial, some authors affirmed that cyst disappearance did not necessarily mean improved clinical outcome. Thereafter, two studies showed a strong association between cysticidal treatment and fewer seizures in NCC patients, a finding that was also questioned due to the non-randomized design of these studies.15,66 During the past few years, placebo-controlled trials have shown that the use of cysticidal drugs not only results in better resolution of lesions but in a lesser risk of seizure recurrence in patients with NCC, providing Class I evidence favoring therapy in these cases.2,22
subarachnoid cysts presenting with seizures. However, because pioneer studies of NCC therapy focused on the number of cysts before and after the trial, some authors affirmed that cyst disappearance did not necessarily mean improved clinical outcome. Thereafter, two studies showed a strong association between cysticidal treatment and fewer seizures in NCC patients, a finding that was also questioned due to the non-randomized design of these studies.15,66 During the past few years, placebo-controlled trials have shown that the use of cysticidal drugs not only results in better resolution of lesions but in a lesser risk of seizure recurrence in patients with NCC, providing Class I evidence favoring therapy in these cases.2,22
The control of epilepsy in NCC patients not only depends on the use of cysticidal drugs, but on the chronicity of the disorder and the presence of brain calcifications. These patients must be treated with an AED regardless of the use of cysticidal drugs. The optimal length of AED therapy has not been settled. A prospective study showed that up to 50% of NCC patients had relapses after AED withdrawal.11 Such patients had been free of seizures during two years, and their brain cysts had been successfully destroyed with albendazole. Prognostic factors associated with seizure recurrence included the development of calcifications, and the presence of both recurrent seizures and multiple brain cysts before cysticidal drug therapy.11,52 Calcified cysticerci are potentially active epileptogenic foci that may cause recurrent seizures after AED withdrawal. Although epilepsy due to NCC may be easily controlled with AEDs, a seizure-free state without medications cannot always be achieved.
Cerebral Malaria
Of the four species of malaria parasites, only Plasmodium falciparum invades the CNS and causes cerebral malaria. Because all species may cause fever associated with delirium or seizures, definition of cerebral malaria requires all of the following conditions to be present: unarousable coma, evidence of acute infection with P. falciparum, and no other identifiable cause of coma.69 Humans acquire the infection when parasites are inoculated through the skin during a blood meal by a female Anopheles mosquito. Malaria is a public health problem around the world. Up to 500 million people are infected by Plasmodium spp. every year, with 3 million fatal cases, most of which occur in African children. Due to increased travel, many cases of cerebral malaria have also been recently recognized in developed countries.63
Pathophysiology
The brain of patients dying from cerebral malaria shows diffuse swelling and small ring hemorrhages in the subcortical white matter. These are related to extravasation of erythrocytes resulting from endothelial damage which, in turn, is caused by the liberation of cytokines and vasoactive substances. Another common neuropathologic finding in cerebral malaria is the plugging of capillaries by parasitized erythrocytes due to an increased adherence of these cells to the endothelium. This causes brain damage as a result of obstruction of the cerebral microvasculature, increased concentrations of lactic acid, and ischemic hypoxia.53
Clinical Manifestations
Cerebral malaria is an acute encephalopathy characterized by headache, seizures, somnolence or agitation that rapidly progresses to stupor and coma, and extensor posturing. Seizures occur in up to 70% of cases, and are most often tonic-clonic generalized, although some patients present with partial seizures.54 Pulmonary edema, renal failure, hypoglycemia, and disseminated intravascular coagulation are common during the acute phase of the disease. Most patients who do not die during the first few days recover without sequelae. Some others are left with hemiplegia, blindness, psychiatric symptoms, speech disturbances, recurrent seizures, and extrapyramidal manifestations.69
Diagnosis
P. falciparum may be seen by examining blood smears with Giemsa stain; repeated examinations may be needed, because parasitemia is cyclic. Dipstick antigen-capture assay may be of diagnostic value in patients with low levels of parasitemia.67 Although the cytochemical analysis of CSF is normal in these patients, a spinal tap is mandatory to exclude other causes of encephalopathy. Neuroimaging studies may show brain swelling,
hypodense areas in the thalamus or cerebellum, small hemorrhages, or dural sinus thrombosis.36
hypodense areas in the thalamus or cerebellum, small hemorrhages, or dural sinus thrombosis.36
Treatment
Because of chloroquine-resistant strains of P. falciparum, quinine is the drug of choice for cerebral malaria.6 The association of quinine plus pentoxifylline may be better than quinine alone for therapy of cerebral malaria in adults. Mefloquine is effective against P. falciparum but has not been evaluated in patients with cerebral malaria. Recent evidence favors the use of artemether as a first-line therapeutic agent for cerebral malaria.43 Symptomatic therapy includes fluid replacement, sedatives, and osmotic diuretics. Corticosteroids are harmful to comatose patients with cerebral malaria and should be avoided. Although phenobarbital is often used to treat malaria-related seizures in endemic areas, the common loading dose of 20 mg/kg may be deleterious for children with cerebral malaria. A recent study suggested that an initial dose of 15 mg/kg followed by two doses of 2.5mg/kg after 24 and 48 hours is safe and effective in these patients.30 Despite therapy, up to 25% of patients with cerebral malaria die. Factors adversely affecting the prognosis include coma, retinal hemorrhages, recurrent seizures, renal failure, disseminated intravascular coagulation, respiratory distress, arterial hypotension, severe anemia, hypoglycemia, altered liver function tests, presence of malaria pigment in peripheral white blood cells, high levels of parasitemia, and co-infection with HIV.8,69
Other Parasitic Diseases of the Central Nervous System
Almost any parasite invading the CNS may cause seizures. These may occur as the result of the pressure effects of a parasite growing within the brain parenchyma (as in the case of cerebral hydatid disease or coenurosis), due to the irritative effects of a larva migrating through the brain (as in gnathostomiasis or sparganosis), or as part of a diffuse encephalopathy (as in patients with cerebral amebiasis or African trypanosomiasis). In any case, seizures may occur during the acute phase of the disease or as a chronic sequelae of the infection.12,58 AED therapy should be promptly started in these cases to stop seizures and to avoid recurrences, irrespective of the specific therapy used for each of these conditions. Duration of AED therapy and the risk of seizure recurrence after AED withdrawal will vary depending on the severity of the initial infection and the occurrence of residual structural brain damage.
Bacterial Infections
Pyogenic Meningitis
Causal agents of pyogenic meningitis vary according to the age and the immune status of the patient, and the route by which the infection gains access to the CNS. Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae are the most common pathogens causing this condition. Bacteria may reach the subarachnoid space by the hematogenous route from a remote infection, or by contiguity from an infection of paranasal sinuses or ears.56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree