Infections of the Spinal Axis
Infections of the spinal axis are an important although uncommon group of diseases that often present with insidious and variably progressive symptoms. The classic clinical hallmarks of infectious disease, such as fever, are variably present. Antimicrobial medical therapy constitutes the cornerstone of rational therapy if diagnosis occurs early. However, delay in diagnosis is common. Surgical intervention makes it possible to obtain tissue or fluid for microbiological diagnosis, drain localized abscesses, relieve compression of neurologic structures, or reconstruct segments of the bony spine destroyed by infection. With advances in imaging, antimicrobial chemotherapy, and the development of safe surgical methods of radical resection and reconstruction of collapsed vertebral segments, there exists the potential to treat and ameliorate the devastating morbidity and mortality that have long been associated with this group of diseases. Spinal infections in children are less completely characterized than those in adults because more of the medical literature consists of case reports and small case series.
81.1 Pyogenic Infections of the Spinal Axis
The pyogenic infections include infections of the vertebral bodies (spondylitis), facet joints (spondylytic arthropathies), intervertebral disks (spondylodiskitis), and epidural space (epidural abscess).1,2 Infections within the dura (subdural empyema, intraparenchymal spinal cord abscess) have been described but are vanishingly rare in the absence of a congenital dysraphic defect, such as a dermal sinus tract, or severe bacterial meningitis. In pyogenic infections, the offending bacteria elicit an inflammatory response that follows a usual acute phase inflammatory reaction (humoral immunity). As such, the predominant leukocytes in the region of inflammation are polymorphonuclear neutrophils. Large populations of lysed polymorphonuclear neutrophils and local necrotic tissue give rise to the purulence for which pyogenic infections are named. The offending bacteria are most commonly staphylococci and streptococci, but gram-negative organisms and Propionibacterium acnes have been identified and implicated.3 There has been a pronounced recent increase in the incidence of postoperative pyogenic infections with methicillin-resistant Staphylococcus aureus (MRSA).4 In at least one-third of cases, an offending organism is never identified. Unlike the microorganisms that cause pyogenic infections, acid-fast microorganisms (e.g., Mycobacterium tuberculosis) and fungi characteristically give rise to a chronic granulomatous type of inflammatory response.2,5
81.1.1 Diskitis
Diskitis in children is a well-recognized although rare clinical entity that may characteristically present three to four times per year in a busy primary pediatric center.6 The disease occurs spontaneously in young children and involves inflammation of the intervertebral disk or cartilaginous vertebral end plate.7,8 Diskitis affects children of all ages, but its prevalence is highest in children younger than 5 years of age.6,8 The incidence of diskitis rises again in adolescents for unknown reasons. Diskitis may occur at any spinal level, but case series indicate that lumbosacral disease occurs more commonly than thoracic disease, which occurs more commonly than cervical disease.8
The etiology of spontaneous diskitis is controversial because negative needle biopsy culture rates of 30 to 50% have been repeatedly demonstrated. Some cases of pediatric spontaneous diskitis have also been observed to resolve without treatment. These observations have prompted some centers to consider it a benign, self-limited process and to characterize childhood spontaneous diskitis as either sterile or infectious. However, the theory of an infectious etiology remains most widely held, in which diskitis results from the hematogenous spread of infection from a remote site, such as the urinary or respiratory tract.6,8 Alternative routes of infection in adults include iatrogenic inoculation during surgery or other invasive procedures and direct extension from adjacent infected tissues.9
Children are at greater risk than adults for the hematogenous spread of infection to the disk space. In children younger than 8 years, a robust network of vessels spans from the cartilaginous vertebral end plate to the annulus fibrosus.6 The nutrient artery of the central canal gives rise to vascular channels that perforate the vertebral end plates and terminate in the disk space. This network of terminal vessels provides a potential route of direct inoculation of the disk space.10,11 The vascular network gradually involutes, with development such that the vascularity is limited to the annulus fibrosus in older children. By adulthood, there is no vascular supply to the disk.7,10,11 The cartilaginous end plates of the vertebral bodies serve as an effective barrier to the transmission of infection from the disk space to the vertebral bodies.11 As such, an infectious process beginning in the disk only rarely spreads to involve adjacent vertebrae. Despite resistance to infection from the disk space, the vertebral bodies remain subject to damage by bacterial enzymes and by increased stress caused by deformed and dysfunctional intervertebral disks. Persistent diskitis may result in destruction of the vertebral end plate and exposure of the vertebral body to infection.8,9,11
Clinical Findings
Diskitis often presents with nonspecific symptoms.6,8,12 Young children or toddlers often present with a limp or a refusal to stand or walk.7,13 The lumbar region is exclusively affected in such children. The assessment can be difficult because young children in pain are often agitated, fearful, and marginally cooperative, and they rarely if ever clearly relate the character or localization of their pain. A toddler with diskitis may crawl normally but refuse to stand and will otherwise appear well. An older child or adolescent may demonstrate a limp or gait impairment.8,12,14 Back pain is the most frequent symptom. Children may describe pain in the hips, legs, or genitals. Dysphagia, neck stiffness, and torticollis may accompany diskitis of the cervical spine. Patients are often afebrile or have a low-grade fever and only rarely appear systemically ill. Vague constitutional symptoms, such as fatigue, weight loss, and appetite changes, may be present but are nonspecific. Therefore, a high index of clinical suspicion for diskitis in any child presenting with low back pain and nonspecific constitutional symptoms expedites the diagnosis and prevents the morbidity that may accompany diagnostic delays.
Children with diskitis do not have neurologic deficits.6 Weakness, numbness, or incontinence suggests a compressive lesion, epidural empyema, or intradural infection.7,8 Tenderness to palpation around the infected level and spasm of adjacent muscles are common physical examination findings in diskitis.14,15 A useful diagnostic examination involves requesting the patient to pick up an object from the floor. Patients with lumbosacral diskitis typically will be unwilling to flex the spine and instead will squat (bend with the knees and ankles and keep the spine in a straight or extended position) to lower the body.13
Diagnostic Tests
Laboratory
The initial laboratory evaluation should include a complete blood cell count with differential (CBC), measurement of the C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR), and blood cultures. If possible, blood cultures should be obtained before the initiation of antibiotic therapy.2,12,15,16
The leukocyte count will often be either normal or slightly elevated. The ESR and CRP are usually slightly higher than normal. Blood cultures can identify the causative organism in as many as 50% of patients with diskitis. This aids in the selection of appropriate antibiotic therapy. If antibiotic therapy has already been initiated and the blood cultures are unrevealing, temporarily withholding antibiotics may be considered in patients who are not declining clinically. In patients with fevers, attempts should be made to collect cultures while the fever is present.6–8,12,14
Radiology
Radiographs of the young child with diskitis (typically demonstrate narrowing of the disk space and may show mild end plate changes if they are obtained several weeks into the illness (▶ Fig. 81.1).8,14,17 Plain spine radiographs can be highly useful in confirming diskitis and may show changes in up to 76% of patients with diskitis, but they are less often abnormal in cases of vertebral osteomyelitis (46%).16 Computed tomography (CT) provides a detailed demonstration of the bony changes that occur in response to disk space infection, which may be difficult to appreciate on plain radiographs.17 CT studies can be obtained relatively quickly, unlike magnetic resonance (MR) imaging, which younger children may be unable to tolerate without sedation.17
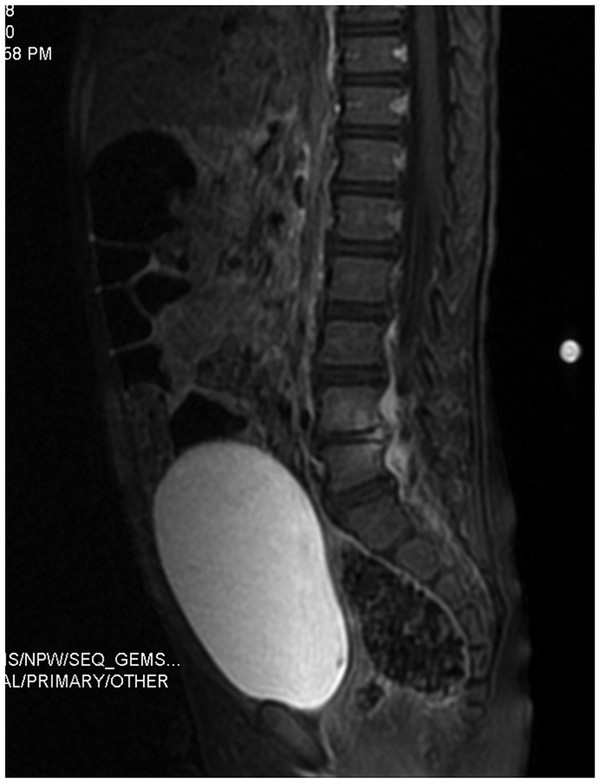
Fig. 81.1 Diskitis. Two-year-old female who presented with a 2-week history of back pain, limping and fever. Post-contrast saggital T1 image shows edema of the L4–5 disc and the adjacent vertebral
MR imaging provides the best definition of the disk and the paravertebral soft tissues and is particularly useful in separating diskitis from osteomyelitis of the vertebral body. Hypointensity within the vertebral body is characteristic on T1-weighted imaging, representing subchondral fibrosis and bone sclerosis. Hyperintensity may be seen in the disk space and paravertebral tissues on T2-weighted sequences, reflecting tissue edema. The administration of gadolinium can help to define the borders of the infectious process. During monitoring after the completion of therapy, the T1-weighted and T2-weighted changes may persist despite clearance of the infection.14,15,17
Treatment
The treatment of diskitis is aimed at resolving the infection, eliminating pain, and preventing the development of a more serious infection, such as pyogenic vertebral osteomyelitis (PVO) or epidural abscess. This can be achieved with nonoperative therapy in almost all pediatric cases. Some centers consider diskitis a benign, self-limited process and provide only analgesia and immobilization while the patient is closely observed for progress. Most centers appear, however, to embrace an infectious etiology and an antimicrobial route of management. If an accurate microbiological diagnosis can be obtained via blood cultures, treatment should be initiated with targeted antibiotics. However, it is common that noninvasive testing fails to identify a causative organism. At that point, the clinical options include initiation of a trial of empiric antimicrobial therapy, image-guided (either CT or fluoroscopy) biopsy, or open surgical biopsy.9 Virtually all series recommend empiric treatment and the reservation of invasive procedures for cases that fail to respond to 7 to 10 days of intravenous antimicrobial treatment.
The most common offending organism in diskitis depends on the clinical setting. In children with diskitis, Streptococcus and Staphylococcus species are frequent. In adults, who typically acquire diskitis as a complication of disk surgery (1 to 2% incidence after elective spinal disk surgery), Staphylococcus species predominate. Empiric treatment with a combination of third-generation cephalosporin and oxacillin/clindamycin has been advocated.14,16,18,19 Recommendations for the duration of treatment for diskitis vary widely, but there is general agreement that therapy should be initiated with intravenous antibiotics for approximately 7 to 10 days, followed by treatment with oral therapy for 2 to 3 weeks until the symptoms resolve and the CRP level normalizes.14,20 Immobilization may contribute to pain control and may be discontinued after the resolution of symptoms9 because instability is not a risk of isolated diskitis.
81.1.2 Pyogenic Vertebral Osteomyelitis/Pyogenic Spondylodiskitis
Although insufficiently treated diskitis may evolve to pyogenic vertebral osteomyelitis (PVO), distinct clinical patterns emerge in the spontaneous, de novo presentation of each disorder.2,24,25 Vertebral osteomyelitis may demonstrate an acute, subacute, or chronic course that varies with both the age of the patient and the identity of the infecting organism. The child with vertebral osteomyelitis is usually older (past the age of 8 years) and more ill-appearing, and the incidence of fever may be higher.2 The incidence of vertebral osteomyelitis peaks in adolescence and again in the later adult years.16,24 About 2 to 7% of all cases of osteomyelitis involve the spine, and pyogenic infection is the most prevalent spondylitis.23,24 Estimates of incidence range from 0.5 to 2.4 per 100,000 overall, but the numbers separate significantly according to age.4,21 In persons younger than 20 years, the incidence is 0.3 per 100,000, but it rises sharply in late adulthood to 6.5 per 100,000.16 Overall, societal incidence rates are increasing, but this is largely due to an increase in the number of elderly patients who are living longer with a greater burden of partially compensated chronic disease.
Like diskitis, spinal osteomyelitis occurs following the hematogenous spread of microbial pathogens from a remote infectious source.1,4,24,26 Infections of the skin, urinary tract, and sinuses are the most frequently implicated, and the majority of spinal infections are preceded by infections elsewhere. Any level of the spine may be affected, but the lumbar region is most frequently involved.1 The Batson venous plexus is implicated in hematogenous spread to the lumbar region from the bladder and pelvis.11,27 Iatrogenic direct inoculation during invasive procedures and spread from an adjacent infection (e.g., psoas abscess) are other mechanisms by which PVO may occur.1,22,24,28
Clinical Findings
PVO is seen more often in older children and young adolescents.4,21,23 The initial presentation is usually nonspecific, and symptoms of pain surrounding the involved spinal level predominate.25 Unlike patients with diskitis, those with vertebral osteomyelitis are more likely to present with fever and to appear systemically ill. If neurologic deficits are present, they are secondary to compression of the neural elements16,23,27,28 and warrant prompt, aggressive evaluation, imaging, and possible intervention.
Diagnostic Tests
Laboratory
The goals of the laboratory work-up should be the same as in diskitis: to identify and isolate the causative organism to guide therapy. Initial studies should include CBC with differential, ESR, CRP, and blood and urine cultures.21,23–25 The CBC often contains an elevated leukocyte count. The ESR and CRP are usually increased (sensitivity, 98 to 100%), and again, serial CRP measurements are useful for monitoring the response to therapy.16,22 Blood cultures (sensitivity, 58%; range, 30 to 78%) and biopsy may be indicated in suspected cases of vertebral osteomyelitis.15,16,21,24 If the blood cultures are negative, then an image-guided or open biopsy (sensitivity, 77%; range, 47 to 100%) may be indicated if there is no response to empiric treatment. Invasive procedures for diagnosis should not be undertaken until a thorough work-up has failed to identify a microbiological diagnosis and there has been no response to empiric therapy. If an invasive procedure is required, image-guided biopsy typically offers the least morbid option for directly obtaining a tissue sample.
Purified protein derivative (PPD) testing may be helpful but is imperfect in diagnosing tuberculosis (TB) as the cause of vertebral osteomyelitis. TB is a rare cause of osteomyelitis in developed regions. Many children in the developing world will have been immunized with bacille Calmette-Guérin (BCG), which is a vaccine against TB made from an attenuated strain of live bovine TB bacilli. If the presentation is subacute or the history includes exposure to TB or Brucella (via the consumption of unsterilized milk), then cultures for Mycobacterium and Brucella may be helpful.2,29 Specific serologic assays, including Bartonella species (“cat scratch”), are available and should be considered when exposure to cats is an element of the clinical history or if the results of other investigations have been negative despite clinical indications of PVO.30
Radiology
Plain film radiographs may reveal focal areas of bony erosion early in the course of the illness (▶ Fig. 81.2). As the disease progresses, destruction of the vertebral body and, potentially, collapse become much more apparent.16,17,31 CT studies can further define the extent of bony erosion and destruction.15,17,31 MR imaging is useful to separate infections of the vertebral body from those that are limited to the intervertebral disk. MR imaging is more sensitive and specific than other modalities, with demonstrated sensitivity of 96% and specificity of 93% in the diagnosis of vertebral osteomyelitis.24,28,30 Vertebral hypointensity on T1-weighted and hyperintensity on T2-weighted sequences represent edema or purulent fluid in the bone marrow or intervertebral disk space. MR imaging, enhanced with the administration of gadolinium, is useful for identifying associated epidural or paravertebral abscesses.16,17
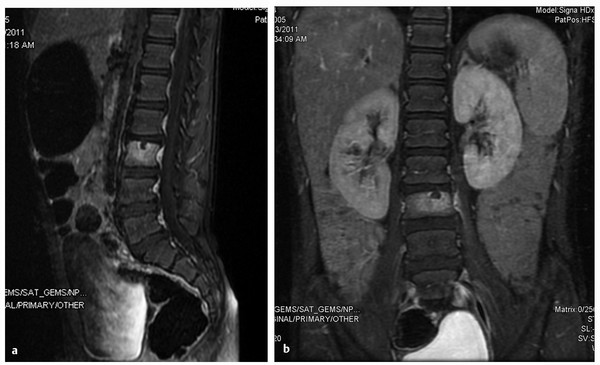
Fig. 81.2 (a,b) Pyogenic vertebral osteomyelitis. Six-year-old male had 2-week history of low back pain with sitting that resolves when lying down. There are no fevers. T1 post-contrast MRI discloses increased signal intensity within the L3 vertebral body with minimal signal change in the L2–3 disc. Blood cultures remained negative and patient did not respond to a 2-week course of IV Clindamycin. He was switched to Vancomycin and Rocephin with subsequent improvement.
Treatment
Long-term antimicrobial therapy with immobilization is the optimal treatment for osteomyelitis.1,16 Empiric treatment, initially with broad-spectrum coverage, is often justified if an organism cannot be identified noninvasively. If cultures obtained during the work-up reveal the causative organism, antibiotic treatment can be tailored appropriately. Current recommendations are for 6 weeks of intravenous antibiotic therapy with possible extension to 8 to 12 weeks depending on the relief of symptoms and reduction of the CRP and ESR.4,21,24,29
81.1.3 Spinal Epidural Abscess
Epidural abscess was first described in 1761 and is recognized as a pyogenic infection of the epidural space. Spinal epidural abscess (SEA) may arise as a result of hematogenous seeding of the epidural space, of trauma or surgery, or of local extension of a soft-tissue abscess complicating PVO.32 It is now widely recognized that diskitis, PVO, and SEA may represent continuous points on a spectrum of infectious illness of the spine; however, the development of a SEA is a serious event that significantly increases the likelihood of permanent neurologic deficit.18,32 Neurologic injury may result from venous infarction due to local thrombophlebitis or from compression of the spinal cord or nerve roots caused by an inflammatory phlegmon.33 The lumbar spine is preferentially involved. The diagnosis can be challenging because patients vary considerably in presentation.34 An SEA may be a neurosurgical emergency, particularly in the setting of progressive neurologic deficit. However, the diagnosis of SEA is often delayed, and the neurologic deficit may be advanced and irreversible at the time of diagnosis. The reported incidence of SEA ranges from 2 to 20 per 100,000, and the vast majority of cases occur in adults.32–34
The majority of cases of SEA in children are individual case reports.18,35,36 SEAs in children are far less common than cranial epidural abscesses, which occur as a complication of common childhood illnesses, such as otitis media and sinusitis.37,38 In a 2001 review of SEA in children, Auletta and John noted that 26 pediatric patients with SEA had been reported in the medical literature, and they further characterized 8 children from their own institution. Most were boys without other illnesses, although 6 had concomitant PVO.39 Observed similarities to adult SEAs were: rarity of the lesions, a prolonged symptomatic period before diagnosis, an etiology of hematogenous spread of infection, and easy detection with MR imaging. Important differences observed included an absence of predisposing conditions and less extensive abscesses in adults yet better outcomes in children.32 The location of the abscesses within the canal also differed significantly in that almost all pediatric SEAs were posterior, whereas a significant number of SEAs in adults were anterior.39 Like the changes in the vascular anatomy of the disk space that impact the epidemiology of diskitis in adults and children, age-related anatomical differences in the vasculature of the epidural space may contribute to the preponderance of posteriorly located abscesses in children. These age-related changes in the vertebral vasculature may affect perfusion and the hematogenous distribution of infecting organisms.39,40
Clinical Findings
The characteristic presentation of adults with the triad of progressive back pain, fever, and progressive neurologic deficit is widely reported in a minority of cases.32 In the large series of adult patients by Rigamonti and colleagues, 29% presented with limited or no back pain and no fever. Many patients had been seen multiple times before the diagnosis was made. Pain that was particularly sharp or lancinating and the presence of a neurologic deficit implicated SEA as more likely than PVO.40 A meta-analysis of the comprehensive world literature identified 915 patients described with SEA. Most patients were in their mid 50s, but infants as young as 10 days old were described. Two-thirds of the patients had fever, and 71% reported back pain. Epidural catheters for regional anesthesia and spinal instrumentation were major iatrogenic sources of SEA.32
Diagnostic Tests
Laboratory
Initial studies should include CBC with differential, ESR, CRP, and blood and urine cultures. As in PVO, the white cell count may be only minimally elevated (mean cell count, 15,000/mm3). The ESR and CRP are uniformly elevated in SEA.32 Culture data to guide antibiotic therapy can often be obtained by sampling during surgical decompression and evacuation.
Radiology
MR imaging is again the diagnostic modality of choice and is highly sensitive for SEA when performed with and without gadolinium contrast (▶ Fig. 81.3). Myelography via lumbar puncture is contraindicated because of the risk for seeding and secondarily infecting the subarachnoid space.
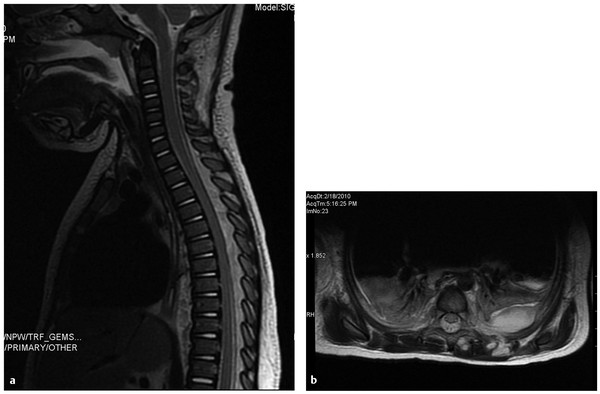
Fig. 81.3 (a,b) Spinal epidural abscess. T2 fat-suppressed MRI images of the spine in a 13- month-old female with MRSA sepsis and positive CSF cultures. There is a faint, dorsal epidural fluid collection from T1 through T9 with enhancement, a left pleural effusion, and paraspinal abscess. The patient was treated with 3 level laminoplasty with irrigation and debridement. She remained neurologically intact and resolved without sequelae.









