12.3.1 Reactive “Functional Hypoglycemia”
Reactive “functional hypoglycemia” is a controversial entity, and it is now known that many organic disorders may result both in fasting and/or reactive hypoglycemia. Many patients present with dizziness or other minor autonomic symptoms within the hours following a meal, but most do not meet the criteria for hypoglycemia, or for Whipple’s triad. Rarely, insulinoma can present as reactive hypoglycemia [37].
12.3.2 Hyperinsulinemic Hypoglycemia
12.3.2.1 Drug-Induced Hypoglycemia
12.3.2.2 Factitious Hypoglycemia Related to Insulin and Insulin Analogues (High Insulin Levels with Low C-Peptide and Proinsulin Levels, Exogenous Hyperinsulinism)
12.3.2.3 Factitious Hypoglycemia Related to Sulfonylureas or Glinides (High Insulin, C-Peptide, and Proinsulin Levels) (Factitious Endogenous Hyperinsulinism)
At the time of symptomatic hypoglycemia, in order to distinguish factitious (or accidental) hypoglycemia from the endogenous abnormal insulin secretion (observed in patients with insulinomas and nesidioblastosis), it is mandatory to collect a plasma sample or a urine collection to measure oral hypoglycemic agents (sulfonylureas and glinides). This diagnosis is difficult and usually suspected in patients whose relatives are diabetic or health professionals [43].
12.3.2.4 Idiopathic Noninsulinoma Pancreatogenous Hypoglycemia Syndrome (Endogenous Hyperinsulinism)
The noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) identifies a group of hyperinsulinemic hypoglycemic patients with unique clinical, diagnostic, surgical, and pathologic features [17, 44]. These patients experience predominantly postprandial hypoglycemia and have nesidioblastosis with islet cell hypertrophy, findings different from those in patients with insulinomas.
12.4 The Histopathological Diagnosis of Insulinomas
The latest 2010 WHO classifies NETs of the pancreas into three categories: (1) well-differentiated endocrine tumors, with benign or uncertain behavior at the time of diagnosis; (2) well-differentiated endocrine carcinomas with low-grade malignant behavior, and (3) poorly differentiated endocrine carcinomas, with high-grade malignant behavior. Most insulinomas are well-differentiated endocrine tumors, WHO group 1; however, occasionally they belong to the WHO 2 or 3 group [45].
For the appropriate histological diagnosis of insulinoma, a detailed macroscopic and microscopic description and an immunohistochemical staining for neuroendocrine tumor markers (chromogranin and synaptophysin), as well as for insulin, proinsulin, and amyloid, are all recommended. However, the immunohistochemical determination of insulin expression by tumor cells does not appear to be an absolute need for diagnosis, as some insulinomas do not stain positively for insulin due to a rapid turnover of insulin secretion from the insulin-producing cells [46]. Evaluation of the mitotic index and Ki-67 index is required to define the grade of the tumor and the related patient prognosis and treatment approach [4].
In 5–10 %, insulinomas may be malignant [47, 48]. Whereas the clinical and biochemical diagnostic criteria of insulinoma do not differ from those of benign insulinomas, the histological diagnosis of malignancy is difficult, and actually the only definite criterion for malignancy is the presence of metastatic disease. However, in clinical practice, a malignant insulinoma is generally found to be a single large tumor, and in most cases, there are synchronous metastases, generally located in regional lymph nodes or in the liver. Suspicion of aggressive tumor behavior, apart from metastases, includes invasion of adjacent organs, a tumor size >2 cm, angioinvasion, and high proliferative activity [49–51].
Rarely, metastatic nonfunctioning pancreatic NETs may change their biological behavior in parallel with tumor progression, with transformation to insulinoma, manifested by a clinical picture of endogenous hyperinsulinemic hypoglycemia (personal experience, unpublished).
12.5 The Role of Anatomical and Functional Imaging in the Diagnosis of Insulinoma
As a rule, imaging should be performed only after the biochemical diagnosis of insulinoma has been established. The role of imaging is to detect the anatomical localization and to stage the tumor prior to surgery [11]. It is known that the majority of insulinomas are solitary and located in the pancreas. They are characteristically small (most being ≤2 cm at presentation) and therefore extremely difficult to localize radiologically [52]. Intraoperative palpation and ultrasound examination of the pancreas are thought to be the best methods to detect insulinomas [53]. Using such methods intraoperatively remains mandatory. However, careful imaging procedure should be performed in an attempt to localize the tumor before surgery, allowing preoperative planning of the surgical procedure according to the size and precise location of the tumor (Whipple’s procedure or middle or distal pancreatectomy, enucleation vs. resection).
The imaging techniques have greatly improved, and the most useful modalities are 3-phase computed tomography (CT), gadolinium-enhanced dynamic magnetic resonance imaging (MRI), and endoscopic ultrasound. Invasive techniques such as selective celiac and mesenteric arteriography, venography, and venous sampling are rarely used, and together with somatostatin receptor imaging and positron emission tomography (PET) with [11]C-5-hydroxytryptophan (5-HTP) as tracer (HTP-PET) or [11]C-l-DOPA (DOPA-PET), they represent complementary techniques in specific situations, such as tumor localization in patients with MEN-1. Unfortunately, there is a wide discrepancy in the use of localization techniques between different centers, as result of the specialist expertise and the availability of imaging equipment [53].
12.5.1 Computed Tomography (CT)
High-definition multi-detector helical CT scan is the first examination recommended to be performed to localize insulinomas; meticulous technique is mandatory [54, 55]. Most insulinomas are small, isodense with the pancreas on pre-contrast images, and then hypervascular on arterial phase images but sometimes are more easily detected on portal venous phase images. More rarely, the tumors are hyperdense in comparison to the pancreas, or have nodular calcification, or appear hypovascular, cystic, or hypodense after injection of contrast medium. Exceptionally, ectopic insulinomas are located in the proximity of the pancreas or of the liver. While the sensitivity of non-helical CT had been 29 %, multi-detector triphasic helical CT scan technique can result in 94 % sensitivity [54, 56].
As the majority of benign insulinomas tend to be small at presentation and, therefore, seldom alter the contour of the pancreas, 3-phase CT should be used to maximize detection. Insulinomas are typically hypervascular, and their appearance is that of a hyper-attenuating lesion in both the arterial and portal venous phases. Liver metastases also tend to be hypervascular, and, therefore, the arterial phase shows the number and size of liver metastases better than the venous phase. The reported sensitivity of CT for the detection of insulinomas is in the range of 30–85 %, depending on tumor size [56], whereas combined 3-phase CT and endoscopic ultrasound may further increase this sensitivity up to 100 % [57].
12.5.2 Magnetic Resonance Imaging (MRI)
MRI has high sensitivities (85–95 %) for the detection of insulinomas and/or insulinoma-related metastases. MRI is superior to CT for the detection of small lesions: the enhancement pattern of insulinomas on MRI is related to their hypervascularity; usually, they are low in signal intensity on fat-suppressed T1-weighted images and moderately high in signal intensity on fat-suppressed T2-weighted images [58–61].
12.5.3 Transabdominal Ultrasound
Transabdominal ultrasound examination is considered to be of low sensitivity in the detection of pancreatic neuroendocrine tumors like insulinomas. Recently, contrast-enhanced ultrasonography has been reported to detect insulinomas in a higher number of patients [62].
12.5.4 Endoscopic Ultrasound
Endoscopic ultrasound (EUS) is currently considered the best preoperative procedure to localize insulinomas with high sensitivity (of 94 %) [63], mainly for lesions located in the head and body of the pancreas and less for tail lesions. Even if recent CT scan techniques lead to localization of most insulinomas, EUS permits a better evaluation of the lesions (especially if they are multiple) and of their proximity to pancreatic ducts and vessels, and it also enables the operator to obtain biopsies [64]. The high spatial resolution of this technique allows the precise anatomical localization of very small lesions. Combined with 3-phase CT, the sensitivity rises to 100 % [64].
12.5.5 Intraoperative Ultrasound and Laparoscopic Intraoperative Ultrasound
Intraoperative ultrasound (IOUS) is mandatory during surgery of insulinomas in order to localize non-palpable tumors. IOUS is also highly useful in defining the relationship of the tumor to the adjacent pancreatic and bile ducts and blood vessels. Intraoperative localization techniques (both careful palpation of the pancreas and IOUS) represent the most reliable way for tumor localization and for proceeding with the appropriate surgical approach (tumor enucleation vs. middle pancreatectomy). Moreover, it is indispensible in patients in whom multiple lesions are suspected, e.g., in MEN-1 patients [65, 66]. In experienced hands, laparoscopic IOUS can identify more than 85 % of insulinomas [67, 68].
Despite the high sensitivity of IOUS, a detailed preoperative examination is necessary to localize insulinomas, to minimize the risk of reoperation, to help in the choice of the surgical technique, and to render laparoscopic surgery as the preferred technique when it appears to be the possible choice to remove the tumor [69].
12.5.6 [111]In-Pentetreotide Scintigraphy (OctreoScan) and Positron Emission Tomography (PET)
OctreoScan is only positive in up to 46 % of benign insulinomas because of the low expression of somatostatin receptor (SSTR) type 2 by insulinomas [70]. In malignant insulinomas, however, the relative distribution of SSTR subtypes is different from benign tumors with a higher rate of scan positivity [71–73].
PET imaging of insulinomas with [18]F-fluorodeoxyglucose ([18]F-FDG) is disappointing, presumably because of their low proliferation rate. Promising results, however, have been obtained using [11]C-5-HTP, [18]F-DOPA, and [68]Ga-DOTA-DPhe [1]-Tyr [3]-octreotide ([68]Ga-DOTATOC) [74, 75].
Recently, there are promising results using a radiolabeled glucagon-like peptide 1 (GLP-1) analogue. Insulinomas are characterized by a very high expression of GLP-1 receptors [70]. Using a radiolabeled GLP-1 analogue ([111]In-DOTA-exendin-4) in six patients with insulinomas, GLP-1 analogue scintigraphy correctly localized the tumor in all patients, whereas CT scan was positive only in 1, MRI scan only in 1, and EUS in 4 [76].
12.5.7 Angiography
Angiography combined with calcium stimulation and transhepatic portal venous sampling (THPVS) was considered the gold standard for insulinoma localization [77]. This technique combines both anatomic and functional localization and screens for an insulin concentration gradient during insulin levels measurement in the right hepatic vein; it may be used in difficult cases [53].
In a recent study [78], the diagnostic accuracy of most methods employed to localize insulinomas in the years 1990–2009 was compared, demonstrating that the multi-detector CT scan employed by an experienced radiologist (with MRI as complementary technique) predicts tumor localization with the highest accuracy, whereas OctreoScan and EUS can be valuable in selected cases, and calcium stimulation may provide an additional functional perspective in those cases not localized by the previously mentioned procedures.
12.6 Therapy of Insulinomas
12.6.1 Surgical Therapy
Surgery remains the only curative treatment of insulinomas, and long-term remission can be achieved by surgery in 95 % of patients [79]. The type of surgery depends on the size and the location of the tumor and on its proximity to anatomical structures (pancreatic duct, common bile duct, splenic and superior mesenteric vessels, and adjacent organs). The entire pancreas should be exposed and explored, using inspection and intraoperative ultrasound, as multiple tumors need to be excluded.
Usually, tumor enucleation is preferred to minimize risks of postoperative pancreatic exocrine deficiency and diabetes mellitus. It should be performed only to excise small (approximately 1 cm in diameter) expectedly benign small tumors on the surface of the pancreas [5]; provided that the surgery is performed by experienced surgeons, the risk of pancreatic fistula after enucleation is not higher than that observed in larger resections of the pancreas [80]. However, when the tumor is anatomically unsuitable for enucleation, central or distal pancreatectomy is safe and effective alternative [68, 81, 82], intraoperative ultrasound IOUS remains mandatory, and rarely, bidigital palpation is required [83]. Importantly, an extensive resection is always the rule for larger size or locally advanced insulinomas that are suspected to be malignant.
Except for pancreaticoduodenectomy (Whipple’s procedure), laparoscopic surgery can be successfully and safely utilized in specialty centers for the majority of pancreatic procedures, with the many advantages in comparison to open surgery [84], although the rate of pancreatic fistulas is not reduced, as it reduces the duration of the hospitalization and the associated morbidity. Laparoscopy must be performed only by experienced surgeons in both advanced laparoscopy and pancreatobiliary surgery. Noteworthy, as intraoperative bidigital palpation of the pancreas is not possible in this situation, laparoscopic ultrasound by an experienced surgeon is mandatory (Fig. 12.1).
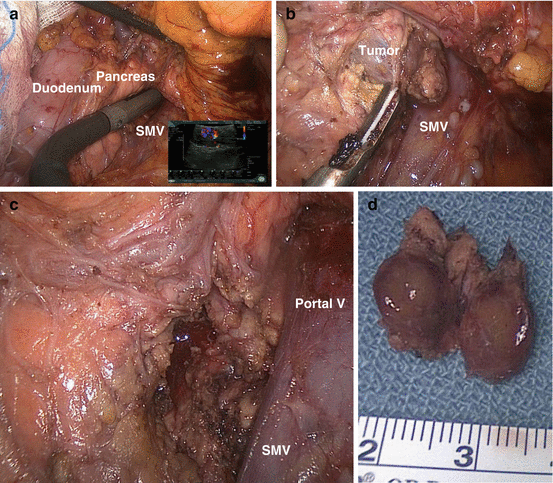

Fig. 12.1
Head of pancreas insulinoma enucleation. (a) Intraoperative laparoscopic ultrasound of the head of the pancreas using a flexible 10-mm probe, showing a 1.2-cm insulinoma. Note the common rich vascularity of the tumor as seen on the Doppler ultrasound in the lower right of the picture. (b) Enucleation of the insulinoma using an ultrasonic energy dissector; SMV, superior mesenteric vein. (c) Post-enucleation, note the tumor bed and the minimal defect in pancreatic tissue. (d) Specimen, the tumor is well encapsulated and completely excised
When no insulinoma is detected at operation, it is recommended to end the procedure and to perform additional investigations in order to localize the tumor. Blind distal pancreatectomy is not recommended [85], due to its short- and long-term morbidity and its frequent failure to achieve disease cure.
12.6.2 Medical Therapy
Dietary consultation is important to prevent prolonged periods of fasting and the development of hypoglycemia and its associated symptoms. Self-monitoring of glucose levels is recommended to insulinoma patients in order to detect asymptomatic hypoglycemia and to prevent occurrence of hypoglycemic spells [86]. The patients should be advised regarding everyday personal safety to avoid loss of consciousness and its possible consequences.
Medical management is reserved only for preoperative control of blood glucose levels, for patients who are unable or unwilling to undergo surgical treatment, or for unresectable metastatic disease.
Continuous glucose infusion may be necessary in some patients, while waiting for surgery or for the effect of other treatments. Vitamin B1 supplementation is recommended for patients with severe recurrent hypoglycemia requiring large infusions of glucose, in order to prevent Wernicke’s encephalopathy [87]. Diazoxide is the most effective drug for controlling hypoglycemia; at a dosage of 50–300 mg/day (up to 600 mg/day), it suppresses insulin secretion by direct action on the beta cells and by enhancing glycogenolysis [88]. However, the disturbing side effects of diazoxide (e.g., fluid retention and edema, weight gain, nausea and digestive intolerance, renal impairment, skin rashes, and hirsutism) limit its use. Verapamil and diphenylhydantoin have also been reported to be helpful in the control of hypoglycemia [89–91]. Glucocorticoids can be effective in refractory cases [92], by decreasing insulin secretion and increasing peripheral insulin resistance. However, their association with well-known adverse effects precludes long-term use of these drugs.
Somatostatin analogues (SSAs) inhibit insulin secretion mainly through their effects on SSTR2A and SSTR5 subtypes, which were found in 70 % of insulinomas [73]; however, in patients with tumors that do not express these receptor subtypes, SSAs may worsen hypoglycemia, possibly by suppression of glucagon secretion [89, 93, 94], and therefore, this therapy should be started in an in-hospital setting. SSAs can achieve normalization of plasma glucose levels in up to 60 % of the patients [95], with few adverse effects, mostly digestive intolerance with diarrhea and steatorrhea that can be managed by pancreatic enzyme supplementation. The dose of octreotide that was found to control the hypoglycemia varied between 50 and 2,000 μg per day, and it had to be determined on an individual basis. A short 100-μg octreotide test, not OctreoScan uptake, was predictive of the long-term efficacy of octreotide treatment on hypoglycemia. This could be explained by the differing affinities of OctreoScan and octreotide for SSTR2 [95, 96].
Interferon alpha may also be beneficial in some selected cases [97].
12.6.3 Specific Treatments for Patients with Malignant Insulinoma
Malignant insulinomas present a dual therapeutic challenge, in terms of both the control of tumor progression and the control of symptomatic hypoglycemia. The course of the metastatic disease is very heterogeneous, with a reported 10-year survival of about 30 % [25]. However, some patients may present with a very slow progression rate, whereas others present with rapid tumor progression and poor survival rate.
Symptomatic treatment of metastatic insulinomas aims at achieving short-term control of the hypoglycemia while awaiting the effects of antitumor treatment or when antitumor treatments do not prove to be effective. All the medications used in benign insulinomas can be employed, but usually, a combination of several hyperglycemic drugs is needed [95].
Since malignant insulinomas are rare, and there is no prospective study to date on this disease specifically, the therapeutic approach is as for other pancreatic neuroendocrine carcinomas. Whenever it is possible, surgery has to be initially considered, aiming at a total excision of all detectable lesions. However, as recurrence is frequent (about 60 %) [47], other treatments should be considered, such as chemoembolization of liver metastases [98], radiofrequency ablation [99], cytotoxic chemotherapy (traditionally with a combination of streptozotocin, doxorubicin, and 5-fluorouracil and, recently, with capecitabine and temozolomide) [100, 101, 102], peptide receptor radionuclide therapy [103–105] (PRRT, especially with 177Lutetium-DOTATATE), or targeted therapies with tyrosine kinase inhibitors (sunitinib) or mTOR inhibitors (everolimus) [106, 107].
The mTOR inhibitor everolimus (Afinitor, Novartis, Switzerland) deserves special mention as it seems to possess a profound effect for the control of both hypoglycemia and tumor progression [108, 109]. Everolimus has recently been shown to improve progression-free survival of patients with well-differentiated progressive metastatic pancreatic NETs and has been approved as a new antitumor therapeutic option for this indication [107]. Long-term administration of everolimus may alter insulin secretion, as well as insulin-mediated peripheral glucose utilization and insulin-mediated suppression of hepatic glucose production, thereby resulting in normo- or hyperglycemia in most of the patients with malignant insulinomas [110]. A recent, largest to date, study in patients with malignant insulinomas [111] clearly demonstrated that 11/12 patients (91 %) experienced a complete resolution of hypoglycemia on everolimus, despite failure of other previous therapeutic modalities. Cardiac and pulmonary tolerance should be carefully monitored in patients treated with everolimus [111].
References
1.
Cryer PE (2009) Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia 52(1):35–37PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






