Intracerebral Aneurysms
Pediatric intracerebral aneurysms are rare, having an overall incidence of 0.6%,1 and are even rarer in infancy.2 The annual incidence of new intracerebral aneurysms in children has not been reported. The annual incidence of hemorrhagic stroke from newly diagnosed intracerebral aneurysms in children is 0.18 per 100,000. Approximately 800 cases of pediatric intracerebral aneurysms have been reported in the literature to date, mostly in the form of case reports and series. However, because of the threat of rupture with potentially devastating neurologic sequelae, it is imperative that pediatric specialists make the appropriate diagnosis and initiate multidisciplinary treatment in a timely manner. Pediatric patients’ extremely long life expectancy following treatment likewise demands that treatment durability be critically assessed.
The mantra in pediatric specialties is that “children are not little adults.” The management of pediatric intracerebral aneurysms embodies this concept. Pediatric aneurysms differ from those in the adult population with respect to aneurysm presentation, location, size, morphology, and natural history. In fact, it has been proposed that pediatric and adult aneurysms are distinct pathologic entities. Whereas aneurysms in adults are more prevalent in women, those in children show a slight male predominance.3 Some have noted a bimodal age distribution in the pediatric age group. In modern series, subarachnoid hemorrhage is not the most common presentation.4,5 Saccular aneurysms are more likely to present with subarachnoid hemorrhage, whereas fusiform aneurysms are more likely to present with symptoms of mass effect.4 When subarachnoid hemorrhage is present, it generally manifests with a low grade on the Hunt and Hess Scale.6,7 The incidence of clinically significant vasospasm after rupture is also significantly lower.8,9 Nevertheless, cerebral infarction secondary to vasospasm does occur in children and should be recognized and treated expeditiously.7,10,11
Aneurysms are most often located at the internal carotid bifurcation.7,8,12,13 Compared with those in adults, pediatric aneurysms are more commonly located in the posterior circulation and are more likely to be giant.3,5,6 Fusiform morphology is relatively more common in children.5,14 Infectious (“mycotic”) aneurysms are generally located either distally in cortical vessels or close to the skull base and cavernous sinus, are usually fusiform, and may be multiple. Pediatric aneurysms demonstrate the capacity to arise de novo and to grow rapidly.3,15,16
66.1 Diagnosis and Initial Management
The initial test in a child with suspected subarachnoid hemorrhage is noncontrast computed tomography of the head. Subarachnoid hemorrhage is the most common pattern of bleeding observed in children with ruptured intracerebral aneurysms. Intracerebral hematoma with or without subarachnoid hemorrhage is rare. In patients with a convincing history to suggest subarachnoid hemorrhage but without CT evidence of blood products, a lumbar puncture should be performed. Blood in the cerebrospinal fluid (CSF) or on a CT scan is further evaluated by vascular imaging. Despite significant advances in CT angiography and magnetic resonance (MR) angiography, conventional four-vessel angiography remains the gold standard for defining the intracranial vascular anatomy. For patients with unruptured aneurysms, MR angiography may be the initial imaging modality. The choice of imaging modality is individualized on a case-by-case basis, with the goal of reducing radiation exposure while obtaining the necessary information to plan subsequent interventions.
Patients who present with a Glasgow Coma Scale score of 8 or less require intracranial pressure monitoring. External ventricular drains are preferred over intraparenchymal pressure monitors because they offer the possibility of CSF drainage to treat elevated intracranial pressure and hydrocephalus and the drainage of blood products in patients with intraventricular hemorrhage. External ventricular drainage is continued peri- and postoperatively. In general, the use of ventricular catheters is continued for the duration of the vasospasm risk period (days 3 through 17 after the bleed). There are cases of children who have reliably improving neurologic examinations with no imaging evidence of ventriculomegaly and a low Fisher grade in whom early removal of the external ventricular drain can be considered. Patients with significant intraventricular blood require ventricular drainage until the CSF is clear of blood products. Before removal of the external ventricular drain, the drain is clamped and the neurologic examination is followed closely. If the examination remains stable, a CT scan of the head is obtained after 24 hours of clamping. If the ventricular size is stable, the ventricular drain is removed. Children who fail this “clamp trial” require ventriculoperitoneal shunting.
66.2 Indications for Surgical Treatment
66.2.1 Subarachnoid Hemorrhage
In a large historical series from 1971, Patel and Richardson reported on 58 pediatric patients who presented with ruptured aneurysms, 21 of which were treated medically.9 Of these patients, 12 died and only 8 survived without neurologic deficits. Storrs et al demonstrated that children with previously untreated subarachnoid hemorrhage had a worse grade when they presented for treatment and subsequently had worse outcomes.12 This was also noted by Sharma et al.7 Therefore, presentation with subarachnoid hemorrhage from a ruptured aneurysm is an absolute indication for treatment. Symptoms of subarachnoid hemorrhage range from the acute onset of a severe “thunderclap” headache, meningeal signs (photophobia, nuchal rigidity), and seizure to a comatose state. Children tend to have a greater capacity than adults to recover after rupture, and we generally maintain an aggressive treatment posture for pediatric patients with aneurysms, even those presenting with poor grades on the Hunt and Hess Scale (▶ Fig. 66.1a–d).
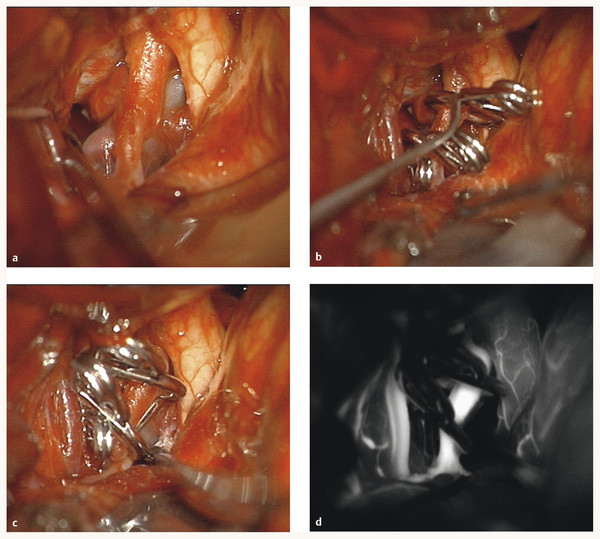
Fig. 66.1 (a) This 5-year-old girl presented with subarachnoid hemorrhage from a giant, thrombotic left internal carotid artery (ICA) aneurysm that was multilobulated. (b) Left pterional craniotomy exposed the aneurysm, which was clipped with two tandem right-angled fenestrated clips around the ICA. (c) A third clip occluded the posterior communicating artery as it exited the aneurysm. (d) Intraoperative indocyanine green dye demonstrated patency of the ICA and its branches.
66.2.2 Mass Effect
Pediatric patients who have an aneurysm with fusiform morphology, more than one aneurysm at presentation, and superimposed medical comorbidities are at increased risk for aneurysm enlargement or the development of new aneurysms.15 Symptoms attributable to mass effect range from headache, dizziness, and cranial nerve palsies to mono- or hemiparesis. Presentation with an enlarging aneurysm and progressive symptoms is an absolute indication for treatment.
66.2.3 Trauma
Traumatic aneurysms are associated with closed head injuries or penetrating head trauma. They are commonly associated with skull base fractures. The injury induces a longitudinal vessel tear with the creation of a false lumen and subsequent dissection. Some are pseudoaneurysms, with injury to all vessel wall layers and hematoma encapsulation, having the imaging appearance of a true aneurysm. The supraclinoid internal carotid artery is the most common location for traumatic aneurysms. This anatomical location is most vulnerable because the proximal internal carotid artery is fixed at the dural entry by the distal dural ring and is more mobile distally where the carotid artery bifurcates to form the anterior cerebral and middle cerebral arteries. Particularly in a patient with a history of head trauma, a noncontrast CT scan demonstrating subarachnoid hemorrhage may be misinterpreted as traumatic subarachnoid hemorrhage. Because trauma can be associated with true or false aneurysms, subsequent vascular imaging is necessary. It has been proposed that traumatic aneurysms may have a more favorable natural history because the artery involved is inherently normal and has the capacity to heal, whereas saccular or fusiform aneurysms arise from abnormal vessels.4 Nonetheless, traumatic dissecting aneurysms that present with subarachnoid hemorrhage are at risk for repeated hemorrhage, and treatment must be considered.
66.2.4 Infection
Infectious or mycotic aneurysms arise in the setting of endocarditis, septicemia, or human immunodeficiency virus infection. Unruptured infectious aneurysms can be treated with appropriate intravenous antibiotics and frequent surveillance catheter angiography to document resolution or stabilization. However, documented growth or rupture of the aneurysm is an indication for definitive treatment (▶ Fig. 66.2a–k).
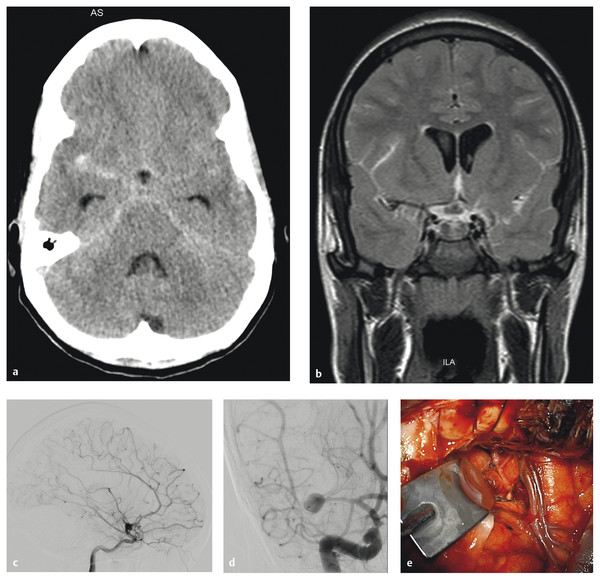
Fig. 66.2 This 17-year-old girl had a history of congenital heart disease and valve replacement surgery. (a) She presented with a subarachnoid hemorrhage as seen on an axial computed tomographic scan, and (b) a mycotic middle cerebral artery (MCA) aneurysm was seen on a magnetic resonance image. The aneurysm enlarged rapidly despite antibiotics, (c,d) as seen on catheter angiography, and she was transferred for surgical care. Note that the superior MCA trunk was occluded at this time. (e) The aneurysm was exposed through a right orbital–pterional craniotomy, (cont.)
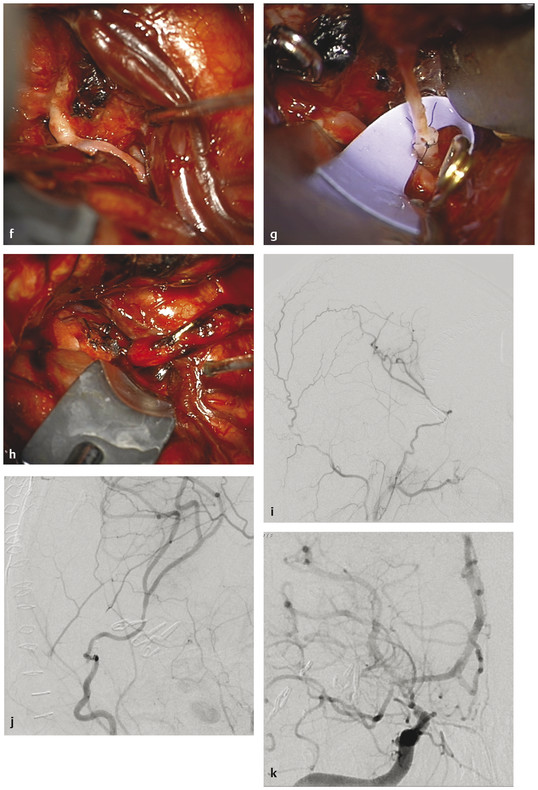
and (f) inspection confirmed a mycotic aneurysm and a superior trunk occlusion. (g) The inferior trunk was revascularized with an end-to-end superficial temporal artery–to–MCA bypass, and (h) the aneurysm was trapped. (i,j) Postoperative angiography demonstrated the bypass filling the inferior trunk and angular arteries, and (k) the aneurysm was excluded completely with preservation of the lenticulostriate arteries.
66.3 Surgical Treatment
Because of the complex and variable morphology of pediatric intracerebral aneurysms, a wide range of microsurgical techniques are required for their treatment. In an institutional series, we reported 13 patients treated initially with microsurgical techniques.5 Treatment techniques included direct surgical clipping, aneurysm trapping alone, trapping with extracranial–to–intracranial bypass, aneurysm excision with anastomosis, and proximal artery occlusion. A small number of patients in the initial microsurgical group required subsequent surgical or endovascular treatment, underscoring the importance of interdisciplinary treatment planning and vigilant follow-up.
The variety of locations of pediatric intracerebral aneurysms mandates that the vascular neurosurgeon be comfortable with the different approaches to these anatomical locations. The standard pterional craniotomy gives access to many of the anterior circulation aneurysms. The pterion, the lesser wing of the sphenoid, and the squamous portion of the temporal bone are removed with a high-speed drill in order to create a smooth, flat surface over the orbit connecting the anterior and middle cranial fossae. This allows an unobstructed view into the carotid cistern once the dura is opened and reflected. The orbitozygomatic craniotomy allows access to basilar tip aneurysms and can be used to gain greater exposure to giant and complex anterior circulation aneurysms. This approach includes the pterional craniotomy supplemented with removal of the orbital rim, orbital roof, lateral orbital wall, and zygomatic arch. Bifrontal craniotomy is used to approach pericallosal artery aneurysms. The medial border of the bone flap crosses the superior sagittal sinus, allowing exposure of the interhemispheric fissure once the dura is reflected medially. Care must be taken with bridging veins during this approach. Posterior inferior cerebellar artery aneurysms and aneurysms of the vertebral artery are approached through a far lateral craniotomy. This involves a C1 laminotomy, lateral occipital craniotomy, and condylectomy.
The treatment of choice for nongiant saccular aneurysms is direct surgical clipping. An aneurysm with a narrow neck and an uncomplicated anatomy can be clipped with a single appropriately sized and contoured clip. For an aneurysm with a broad neck or more complex anatomy, multiple clips oriented in intersecting, stacked, or overlapping configurations may be required. Giant saccular and fusiform aneurysms can also be treated by surgical clip reconstruction. In this technique, the arterial lumen is reconstructed with multiple surgical clips when a large size, wide neck, and/or abnormal branches prevent simpler clip applications. Complex clip reconstruction often requires proximal and distal temporary clips to soften the aneurysm, and it may be necessary to open the aneurysm to deflate it or remove thrombus intraluminally. Aneurysms not amenable to direct clipping may need to be trapped and bypassed ▶ Fig. 66.3a–m). A small subset of aneurysms can be excised with end-to-end anastomosis of the normal artery ends; however, this requires enough slack on either end for the ends to be brought together and sutured without tension.
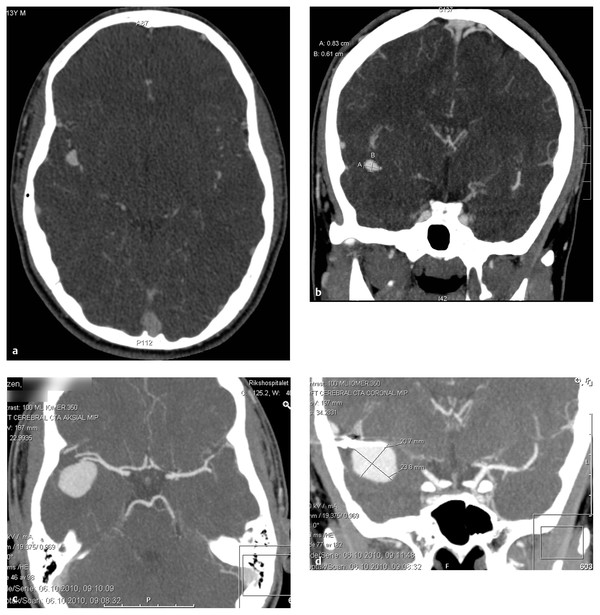
Fig. 66.3 This 15-year-old boy presented with a recurrent, giant distal middle cerebral artery (MCA) aneurysm. (a,b) He had presented 2 years earlier with a small MCA aneurysm arising from a bifurcation in the superior division of the MCA along the insular M2 segment. He underwent right pterional craniotomy and direct clipping in Norway, with no surgical complications. (c,d) Follow-up revealed aneurysm recurrence with marked enlargement, (e,f) as seen on computed tomographic angiography (cont.)









