Intracranial and Extracranial Hematomas in Children
There are many causes of intracranial and extracranial hematomas in children; this chapter focuses on the most common and the most relevant to neurosurgical intervention.
55.1 Basic Pathologic and Pathophysiologic Considerations
Intracranial hematomas present a major risk for death and disability because they increase intracranial pressure (ICP) and displace brain. The volume within the cranial compartment is fixed (Monro-Kellie hypothesis); therefore, any increase in one of the principal components (brain, blood, cerebrospinal fluid [CSF]), or the addition of another component (e.g., hematoma) must be compensated for by a change in one of the other components—initially, CSF and venous blood. If this compensation is inadequate or exhausted, ICP increases and brain shift occurs, leading to global or focal ischemia, brain distortion, focal deficits, and decreased consciousness. Of great clinical importance is the nonlinear relationship between increased intracranial volume and pressure. When the brain is able to compensate for changes in volume, there is little change in ICP; however, once the compensatory capacity, or compliance, of the intracranial space is exhausted, ICP increases dramatically with any further small increase in volume. Therefore, an awake patient with an intracranial hematoma remains at risk for sudden deterioration and should be treated with caution and care, even when he or she is neurologically intact. Open fontanels and sutures in infants and young children offer limited protection against an acute rise in pressure.
The risk for an intracranial hematoma depends on the etiology, location, speed of accumulation, and eventual size. Hematomas of arterial origin accumulate most rapidly. Posterior fossa hematomas are particularly dangerous because of the relatively contained infratentorial space, resultant pressure against the brainstem, and occlusion of the fourth ventricle leading to hydrocephalus. The condition of the brain is important—large intraparenchymal contusions and/or brain swelling may lower the threshold for removing relatively small extra-axial hematomas. Conversely, if a lesion has minimal mass effect in a patient who is systemically unstable (e.g., a patient with polytrauma), is medically unwell (e.g., a patient with cardiac disease), or has a coagulopathy, an initial conservative approach may be more prudent. Acute evaluation must also consider the potential dynamic element; very early imaging may underestimate the enlarging hematoma, and the initial clinical examination must be supported by ongoing and close neurologic monitoring to detect any delayed deterioration in a timely manner.
55.2 Imaging
Plain skull radiographs rarely contribute to the initial assessment; although linear fractures may indicate an underlying hematoma, clinically important intracranial pathology in trauma may be present with no fracture, and therefore plain skull radiographs are not reliable indicators for further imaging. The exceptions are suspected nonaccidental injury and the follow-up of potential growing skull fractures. Computed tomography (CT) of the head is the primary imaging modality in most circumstances. Some institutions prefer magnetic resonance (MR) imaging, even in emergencies, but this requires controlled sedation or general anesthesia in younger children, and it may not be logistically feasible in an emergency setting. There are clear benefits of MR imaging, though, particularly for nontraumatic hematomas, the underlying cause of which can be more thoroughly investigated. The characteristics of blood products on MR imaging depend on the MR imaging sequence and time after bleed. In many ways, head CT is simpler and quicker, and it remains the standard for emergency evaluation. However, concerns about the radiation dose delivered to the developing brain are growing.1,2
The most important imaging characteristics to assess are the following: hematoma size, regional location (left or right, posterior fossa or supratentorial); layered location (epidural, subdural, parenchymal, subarachnoid/intraventricular); local mass effect and distortion of the brain; comorbid brain swelling or hydrocephalus; and any additional pathology associated with the cause of the hematoma. The size of the hematoma can be estimated by the ABC/2 rule: A measures the longest diameter of the hematoma, B measures the diameter perpendicular to the A line, and C measures the number of slices over which the hematoma is visible multiplied by the slice thickness in centimeters. If the image slice contains 75% or more of the hematoma volume at its largest, it is counted as 1 full slice; if 25 to 75%, it is counted as 0.5 slice; if less than 25%, it is not counted. The three measurements are then multiplied and divided by 2 to derive a volume in milliliters.3 The rule works best with intraparenchymal hematomas but can be adapted for extra-axial hematomas. Midline shift is measured on axial CT at the third ventricle, septum pellucidum, or pineal gland; it may be greater than the width of the hematoma when hemispheric brain swelling is present. Hematomas are hyperdense on head CT but may be isodense if hyperacute (as on a very early scan), and they lose their density over time. Subdural isodense lesions may be difficult to appreciate before the typical hypodensity of chronic hematomas develops.
55.3 Extracranial Hematomas
Extracranial hematomas seldom require surgery. They typically occur after some form of trauma.
55.3.1 Childhood Posttraumatic Scalp Hematomas
Scalp hematomas are common after childhood trauma and seldom are of any surgical importance other than indicating the severity and location of the trauma. They may overlie a skull fracture. These hematomas may be large because of the vascularity of the scalp and easy accumulation. This is of particular importance in infants, in whom the blood lost in the hematoma may cause a significant drop in the hemoglobin level. Scalp hematomas sometimes increase in size as they resolve and the clot liquefies. Rarely, they may become secondarily infected. “Raccoon eyes” and the Battle sign (mastoid bruising) may indicate a fracture of the base of the skull.
55.3.2 Neonatal Subgaleal (Subaponeurotic) Hemorrhage
Subgaleal (subaponeurotic) hemorrhage can be life-threatening in neonates because of the wide potential space in which the clot can spread. Unlike the cephalohematoma, it is not limited by suture lines, so that ongoing bleeding may lead to severe anemia and hypotension; therefore, mortality is high. It presents with a diffuse scalp swelling or fluctuant mass, crosses suture lines, and shifts when the child’s head is repositioned. It may be associated with a skull fracture or with rupture of an interosseous synchondrosis or emissary veins between the subdural and subgaleal spaces.4 Instrumented delivery is a risk factor. Occasionally, surgery may be required to control the bleeding vessels.
55.3.3 Cephalohematoma
Cephalohematomas are localized subperiosteal clots caused by birth trauma, more often associated with instrumented delivery. The hematoma is limited by the adherence of the periosteum at skull sutures and most commonly occurs parietally, sometimes bilaterally. Occasionally, it is significant enough in size to decrease the infant’s hemoglobin level, but this is rare; most are asymptomatic and resolve spontaneously over a few weeks. Cephalohematomas sometimes enlarge and/or calcify over time, leaving an unsightly bump in the parietal region. This can be corrected at a later age. Uncommonly, they may become infected, leading to meningitis or osteomyelitis. This rarely requires a diagnostic tap—possibly if there is an increase in size, the development of erythema and/or fluctuance, or delayed resolution or relapse of clinical signs of infection.5 Sometimes, there is an associated epidural hematoma at the same site that is in communication with the extracranial lesion. Rarely, a traumatic cephalohematoma may occur in an older child. A cavernous hemangioma of the skull may occasionally look like a cephalohematoma (▶ Fig. 55.1).
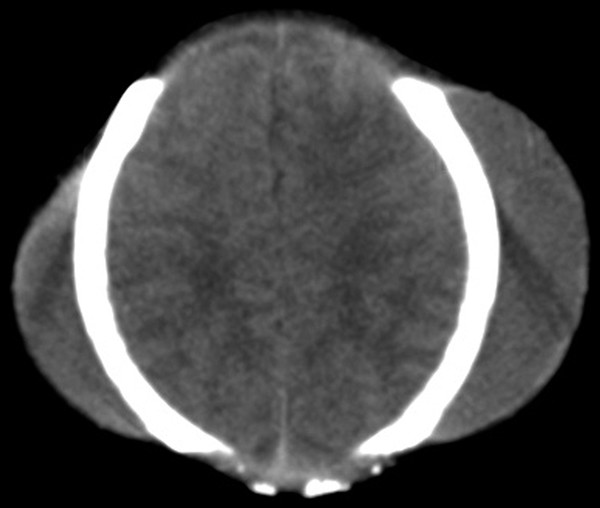
Fig. 55.1 Cephalohematoma. Computed tomographic scan of the head showing the typical appearance of bilateral cephalohematomas.
55.4 Intracranial Hematomas
Intracranial hematomas may be traumatic or spontaneous. Many of the principles that apply to the former apply equally to the latter.
55.4.1 Traumatic Hematomas
Basic Surgical Principles
The removal of an intracranial hematoma in trauma usually constitutes an emergency. The patient is transferred to the operating room after resuscitation and head CT. The anesthesiologist must prepare for ongoing resuscitation and potential hemorrhage; large-bore intravenous access and blood cross-match may be needed; clotting must be checked. The head of the operating table is elevated to approximately 20 degrees. Hypertonic saline or mannitol may be used to decrease ICP during preparation for the craniotomy, as may temporary mild hyperventilation, while a high inspired fraction of oxygen is maintained. Blood pressure is aggressively maintained, and at no point should the patient be allowed to become even slightly hypotensive. The patient is positioned and the skin flap is planned according to the type of craniotomy planned.
As a general principle, the skin incision and corresponding bone flap should be large enough to allow maximal decompression and the control of bleeding points. For hematomas over the convexity, a large trauma flap (question mark or reverse question mark) incision is performed, for frontal lesions a bifrontal skin incision is made, and for posterior fossa lesions a linear midline or paramedian incision is used. For localized convexity epidural hematomas, a linear incision is sometimes sufficient. For trauma flaps, always ensure that the base of the flap is larger than the length to ensure viability. If the patient’s condition is deteriorating rapidly, priority is given to temporal decompression to allow maximal space at the level of the tentorial hiatus before the rest of the craniotomy is completed. Hemostats are used to minimize bleeding from the scalp edges. When turning the scalp flap, take care to not create a sharp fold that reduces blood flow to the flap, especially in very young children. When the bone flap is created, the craniotome is angled to create a bevel that improves contact of the flap when it is replaced. If the dura needs to be opened, ensure that brain swelling has been maximally controlled first (see below). After the hematoma is out, spend time ensuring adequate hemostasis to avoid returning to the operating room to evacuate a re-collection. A subgaleal drain may be used postoperatively (and only for a short period) but should never be a substitute for poor hemostasis. Further details about specific hematomas are discussed in their relevant sections below.
Whether the bone flap is replaced primarily after evacuation of acute subdural or intraparenchymal hematomas depends on the circumstances. If there is substantial brain swelling or increased ICP (with a monitor in situ), it may be safer to leave the bone flap out so that brain swelling may be controlled in the intensive care unit. In this case, the dura may be enlarged with a pericranial patch. These decisions should be individualized. If the bone is left out, it should be kept in a bone freezer in sterile conditions, or in an abdominal pocket, and replaced as soon as brain swelling has settled (within 4 to 6 weeks if possible). If there are no concerns after the hematoma is evacuated, the bone is replaced.
In general, a lower threshold is used for the evacuation of posterior fossa hematomas for the reasons previously discussed. Mass effect is judged by the effacement and displacement of the fourth ventricle and cisternal spaces and by distortion of the brainstem. This is of particular concern when hydrocephalus is already present. If there is no hydrocephalus and a conservative approach is chosen, repeat the imaging relatively early to detect any increase in the size of the hematoma or the development of hydrocephalus; in these patients, acute coma or apnea may develop. Craniectomy is favored above craniotomy in this location.
Epidural Hematomas
Epidural hematomas (EDHs) are usually secondary to a skull fracture that lacerates a meningeal vessel or venous sinus, or that causes venous blood to accumulate from the fracture edges; however, occasionally there are no associated fractures. In infants, EDHs are less common because the dura is relatively well attached to the skull, and the meningeal vessels are not encased and so are relatively easily displaced rather than torn. An EDH is in many ways the most lethal complication of head trauma that is amenable to surgery, with the potential for complete recovery. An EDH may be associated with very little parenchymal brain injury sustained during trauma but may lead to death due to rapidly developing mass effect. Therefore, the critical determinant is the time to surgery. Classically, a lucid interval may occur; the patient recovers from the initial concussive trauma but then deteriorates as the growing hematoma distorts the brain. Overall mortality is about 5% in children, but this depends on the associated findings and presenting condition.6
Although an EDH occasionally may be treated conservatively, the indications for this approach are very specific—the requirements are minimal mass effect of the hematoma (maximum thickness usually < 1 to 1.5 cm), an awake patient, close neurologic observation, and repeated imaging. No formal guidelines exist for children, but the adult literature advises removal of any EDH with a volume of 30 cm3 or more.6 When the mass effect of an EDH is evaluated, particular attention must be paid to its location, midline shift, and clinical status of the patient (Glasgow Coma Scale score and focal signs).
The typical appearance on head CT is of a medially convex hyperdense collection. Although EDH commonly occurs frontally or on the convexity, occasionally it occurs in the more dangerous temporal fossa and posterior fossa. The choice of scalp flap is determined by the location and size of the hematoma. A conventional large trauma flap is appropriate for large lesions; however, a focused and quick vertical incision may be used for a patient with a localized clot or for a patient who has deteriorated rapidly and for whom emergent decompression is the priority. The craniotomy for an EDH should be large enough to evacuate the hematoma and cover the area of probable origin. The surgeon must be particularly careful when fractures cross, or are close to, dural venous sinuses. If so, there must be adequate preparation for potential hemorrhage and a plan to control the sinus. Similarly, depressed fractures must be elevated carefully in cases with significant parenchymal injury, lacerated pial vessels, or proximity to venous sinuses. Hemostasis at the end of surgery must be meticulous. The scalp has been traumatized and tends to be inflamed and prone to oozing. Clotting may be impaired. Bleeding bone edges, especially at fractures sites, should be carefully occluded with bone wax. The use of oxidized cellulose and dural tack sutures at the bone edges of the craniotomy, and perhaps a central hitch suture, may reduce the risk for reaccumulation of the hematoma. If these are used, the surgeon must ensure that placing the suture does not cause a subdural bleed. The bone is replaced and secured with plates and screws (▶ Fig. 55.2).
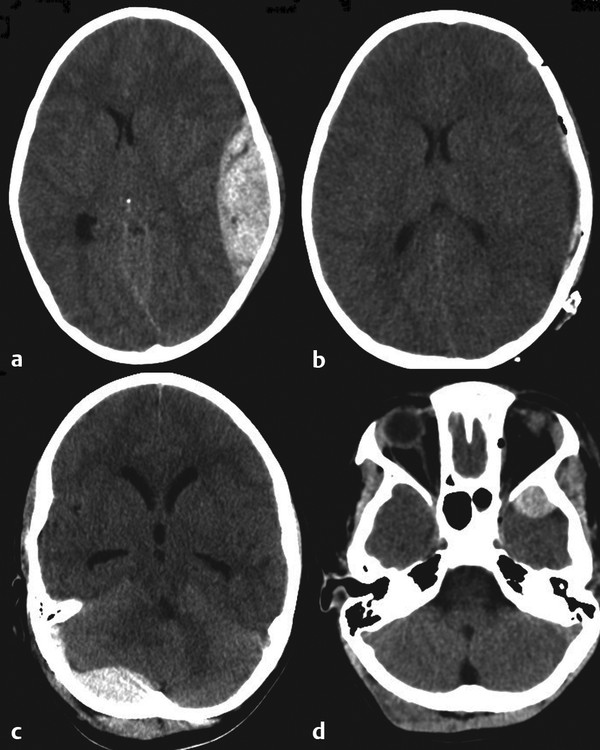
Fig. 55.2 Various epidural hematomas. (a) Left-sided temporoparietal epidural hematoma. (b) Corresponding post-evacuation image. (c) Posterior fossa epidural hematoma. (d) Left temporal tip epidural hematoma.
Subdural Hematomas
A subdural hematoma (SDH) may be acute (hyperdense on CT), subacute (3 to 14 days, isodense on CT), or chronic (more than 14 days, hypodense on CT). However, although the imaging characteristics may vary during this period, many define an acute SDH up to 14 days after injury.7 An SDH is usually located over the convexity of the hemisphere but may occasionally be interhemispheric, on the tentorium, or in the posterior fossa.
Acute Subdural Hematomas
Acute SDHs are more likely than EDHs to be associated with underlying brain injury (contusions, brain swelling), so the initial presentation and prognosis tend to be worse; mortality is reported to be as high as 40 to 60%.7 The hematoma usually arises from a tear of a cortical bridging vein but may also decompress from a superficially located intraparenchymal lobar hematoma (“burst lobe”). Typically, there is an acceleration/deceleration component to the injury (motor vehicle accidents or falls from a height). Acute SDHs (or acute-on-chronic hematomas, or hematomas of differing ages) in infancy should always raise the suspicion of nonaccidental injury, particularly when there is a discrepancy with the history. SDHs that occur after birth trauma should be considered separately (see below).
The typical appearance of an acute SDH on head CT is that of a crescentic (convex outward), hyperdense clot, often with underlying brain swelling. Over time, the density of the clot decreases; however, a hyperacute clot may also be relatively hypodense. The indications for evacuation of an acute traumatic SDH must be individualized to the patient (age, size and location of the hematoma, level of consciousness and focal signs, associated lesions). However, most clinicians would agree that surgery should be considered for a hematoma with a thickness of more than 5 mm. Official recommendations for adults suggest a thickness of 10 mm or more and/or a midline shift of 5 mm or more.7 If the thickness is less than that, surgery is still recommended if there is clinical deterioration.
At surgery, the brain tends to be swollen and hyperemic. The bone flap must be large enough to expose the point of bleeding. If the dura is very tense before opening, it may be prudent to evacuate the clot initially through sequential slits in the dura. Widely opening the dura without control of brain swelling may precipitate the herniation of a congested, hyperemic brain. If the brain is known to be swollen before surgery, the anesthesiologist can take the following measures to assist with the control of ICP: elevating the level of the bed, providing good analgesia and anesthesia, administering hypertonic saline/mannitol at the start of surgery or just before, avoiding hypertension, controlling the CO2 level, and temporarily hyperventilating the patient when the dura must be opened (at 100% FiO2). These maneuvers in combination are usually successful in controlling brain swelling, at least for the time period required to open the dura, evacuate the clot, and place an expanded duraplasty. The clot must be evacuated gently under direct vision because sometimes thrombosis has occurred at the point of bleeding but suction of the clot from the vessel may cause rebleeding. This is particularly dangerous when clot beneath the dura, where it is not fully exposed, is suctioned. Caution should be exercised, with gentle suction and wash of the clot. If the brain is swollen, a duraplasty with pericranium or a dural substitute is used to close the dura, and the bone should be left off.
Chronic Subdural Hematomas
Chronic SDHs are most commonly secondary to trauma, ventricular shunts, or surgery. A special consideration is the SDH that occurs after nonaccidental injury. The latter condition may present acutely but is commonly seen in a patient with a background of previous injury that has resulted in brain atrophy and bilateral SDH collections with different densities because the bleeds occurred at different times. The prognosis is often poor as a consequence of multiple episodes of injury, a delayed presentation, and the vulnerability of the young child at presentation. Anticoagulants are a risk factor after minor head injury. Subdural collections that develop after overdrainage due to a ventricular shunt contain CSF or blood, or a mixture of both. Although these collections sometimes resolve spontaneously, most cause a progressive mass effect or calcify over time. Treatment depends on the size of the collection and the type of shunt used. In general, the drainage of significant collections and upgrade to a higher-pressure valve, programmable valve, or antisiphon device may be indicated.
Chronic SDHs must be distinguished from the benign extracerebral subarachnoid collections of CSF seen in external hydrocephalus and the subdural effusions associated with meningitis, especially Haemophilus meningitis. Nonaccidental injury must also be distinguished from glutaric aciduria type 1, a rare metabolic disorder whose radiologic signs may mimic those of the former.8
In a chronic SDH, a vascularized membrane develops over the subdural collection as a consequence of the brain’s inflammatory response. Repeated bleeds from the vascularized membrane may progressively enlarge the collection. Treatment depends on the underlying condition but may involve a subdural tap through the lateral aspect of an open fontanel, bur hole drainage and irrigation, or placement of a subdural drain or shunt, with correction of the underlying abnormality where appropriate (▶ Fig. 55.3).
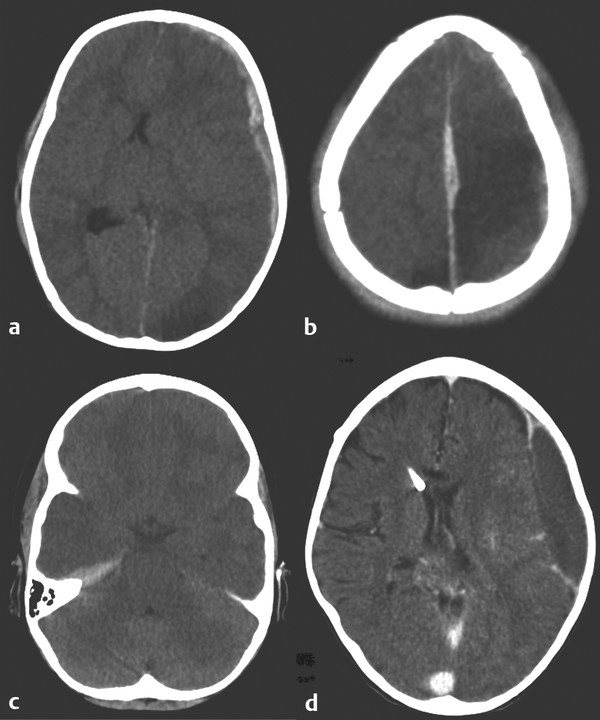
Fig. 55.3 Various subdural hematomas. (a) Swollen brain after trauma, with a left-sided acute subdural hematoma, midline shift, and left occipital hypodensity. (b) Interhemispheric subdural hematoma and bilateral hypodensities. (c) Subdural hematoma on the right tentorium. (d) Chronic subdural hematoma after cerebrospinal fluid overdrainage related to ventriculoperitoneal shunting.









