CHAPTER 43 INTRACRANIAL HEMORRHAGE: ANEURYSMAL, IDIOPATHIC, AND HYPERTENSIVE
Bleeding in the cranial space, or intracranial hemorrhage (ICH), often results in disastrous consequences for patients. ICH can be classified in terms of location, being either intraparenchymal or within spaces such as the subarachnoid, ventricular, subdural, or extradural space. Bleeding may be spontaneous or traumatic; sometimes no cause is found. It should be remembered that intracranial vascular abnormalities can also manifest without bleeding; early signs may be caused by compression of surrounding anatomical structures.
The effect of ICH on the general population can be fully grasped when viewed within the broader category of stroke, currently the leading cause of neurological deficit in the world and the third leading cause of death in the United States, accounting for 15% of all U.S. deaths annually.1 ICH accounts for 10% to 17% of all strokes, and the rate of mortality from ICH is considerably higher than that from nonhemorrhagic or ischemic stroke; some studies quote a mortality rate as high as 90%.2 This chapter focuses on ICH with particular regard to cerebral aneurysm and hypertensive and idiopathic hemorrhage.
ANEURYSMAL INTRACRANIAL HEMORRHAGE
Epidemiology
Cerebral aneurysm has been recognized as a cause of human illness since the end of the 19th century, but the actual incidence is difficult to estimate. In autopsy studies, the prevalence ranges from 0.2% to 7.9%. Other studies indicate a prevalence of 5%.3 The ratio of ruptured aneurysm to unruptured (incidental) aneurysm is 5:3 to 5:6 (rough estimate is 1:1; i.e., 50% of these aneurysms rupture).4 Only 2% of aneurysms manifest during childhood.5
Etiology
The exact pathophysiology of the development of aneurysms is controversial, but abnormal vascular anatomy and flow dynamics are important factors. In contrast to extracranial blood vessels, there is less elastic in the tunica media and adventitia of cerebral blood vessels, the media has less muscle, the adventitia is thinner, and the internal elastic lamina is more prominent.6,7 Outpouching is more likely to occur because of this, but it is also noteworthy that aneurysms are usually found at vessel bifurcations at sites of greatest flow. This, together with the fact that large cerebral blood vessels lie within the subarachnoid space with little supporting connective tissue,8,8a may predispose these vessels to the development of saccular aneurysms. Aneurysm formation is more common in women and in smokers. Beyond the third decade in particular, SAH is usually caused by rupture of a cerebral aneurysm. Other than size of aneurysm alone, the “aspect ratio” (aneurysm depth to aneurysm neck) has also been shown to be a potentially useful predictor of rupture (Figs. 43-1 and 43-2).9
The etiology of aneurysms may be any of the following:
 “Atherosclerotic” or hypertensive, the presumed etiology of most saccular aneurysms; probably interacts with congenital predisposition
“Atherosclerotic” or hypertensive, the presumed etiology of most saccular aneurysms; probably interacts with congenital predispositionOf all saccular aneurysms, 85% to 95% are located in the carotid system, with the following three most common locations: (1) the anterior communicating artery (ACom) for 30% (aneurysms in the ACom and anterior cerebral artery are more common in male patients); (2) the posterior communicating artery for 25%; and (3) the middle cerebral artery for 20%. Of the remaining saccular aneurysms, 5% to 15% are located in posterior circulation (vertebrobasilar); 10%, at the basilar tip (most common), followed by the basilar artery-superior cerebellar artery junction, basilar artery-vertebral artery junction, and anterior inferior cerebellar artery; and 5%, on the vertebral artery (vertebral artery-posterior inferior cerebellar artery junction most common). Twenty percent to 30% of patients with aneurysm have multiple aneurysms.10
Manifestation of Aneurysms
Major rupture is the most frequent manifestation leading to SAH. Clinical features may vary from sudden headache to a moribund comatose state. Neurological deficits, if present, depend on location and size of the hematoma. Signs of meningism with photophobia, neck stiffness, and vomiting are often seen. Progressive neurological deficit or decreasing con scious level may occur as a result of mass effect from a growing hematoma or the development of hydrocephalus. Ruptured cerebral aneurysm may be accompanied by the following:
 Intracerebral hemorrhage, which occurs in 20% to 40% of affected patients (more common with aneurysms distal to the circle of Willis; e.g., middle cerebral artery aneurysms).
Intracerebral hemorrhage, which occurs in 20% to 40% of affected patients (more common with aneurysms distal to the circle of Willis; e.g., middle cerebral artery aneurysms).Intraventricular hemorrhage occurs with 13% to 28% of ruptured aneurysms in clinical series (higher in autopsy series)11 and may lead to obstructive hydrocephalus. The presence of intraventricular hemorrhage is an indicator of poor prognosis11 (64% rate of mortality); ventricular size on admission is the most important prognostic factor (large ventricles being worse). Patterns that may occur include the following:
 ACom aneurysm, which tends to produce intraventricular hemorrhage by rupture through the lamina terminalis into the lateral or anterior third ventricles.
ACom aneurysm, which tends to produce intraventricular hemorrhage by rupture through the lamina terminalis into the lateral or anterior third ventricles. Distal basilar or carotid terminus aneurysms, which may rupture through the floor of the third ventricle.
Distal basilar or carotid terminus aneurysms, which may rupture through the floor of the third ventricle.Other Possible Presentations
Mass effect can give rise to possible “warning signs”: for example, giant aneurysms can include brainstem compression, which produces hemiparesis and cranial neuropathies; cranial neuropathy (average latency from symptom to SAH was 110 days) can include non-pupil-sparing third nerve palsy produced by expanding posterior communicating artery aneurysm; rarer cranial neuropathies produce visual loss as a result of compressive optic neuropathy12 (ophthalmic artery aneurysms) and chiasmal syndromes (ophthalmic, ACom, or basilar apex aneurysms). Facial pain syndromes in the ophthalmic or maxillary nerve distribution that may mimic trigeminal neuralgia can occur with intracavernous or supraclinoid aneurysms.13,14
Also, intrasellar aneurysm may produce disturbances in the endocrine system.15 Warning or sentinel hemorrhage (minor bleeding) may occur; this condition has the shortest latency (10 days) between symptom onset and SAH.16 Headache without hemorrhage may occur; this may be acute, severe, and “thunderclap” in nature, sometimes described as the “worst headache of my life.” It may be caused by aneurysmal expansion, thrombosis, or intramural bleeding17 (all without rupture). Alternatively, it can be chronic, having usually been present for more than 2 weeks, is unilateral (often retro-orbital or periorbital) in about one half of cases, and is possibly caused by dural irritation. In the other one half of cases, it is diffuse or bilateral, possibly caused by mass effect that produces raised intracranial pressure. Asymptomatic aneurysm may be discovered incidentally on angiography, computed tomographic (CT) scan, or magnetic resonance imaging obtained for other reasons.
Conditions associated with aneurysms include the following:
Treatment
Exclusion of the Aneurysm
Traditionally, aneurysmal occlusion was achievable only with craniotomy and dissection of the aneurysm in the basal cisterns and surgical clipping of the aneurysm neck. However, endovascular occlusion with platinum coils is being increasingly used as an alternative to craniotomy. This trend has been influenced by the results of the International Subarachnoid Aneurysm Trial (ISAT), which concluded that coiling is a safer treatment option than surgery (Molyneux et al, 2002).18 This remains a controversial area, however, and long-term studies investigating the risk of rebleeding (i.e., efficacy) after coiling are particularly required.
Cerebral Vasospasm
Cerebral vasospasm is constriction of the cerebral arterial vasculature that leads to delayed cerebral ischemia. The pathophysiology is poorly understood. Traditionally, rebleeding was the major concern after rupture of a cerebral aneurysm. However, the general shift to early occlusive therapy has minimized the effect of rebleeding. Indeed, except for the initial hemorrhage, cerebral vasospasm is now recognized as the most significant cause of death and disability, killing 7% of patients and leading to severe disability in another 7%.19 It is present in 20% to 30% of cases of aneurysmal SAH and is seen in 30% to 70% of angiograms at day 7 after SAH with and without clinical deficit. Vasospasm typically occurs at days 3 to 5 but has been reported up until day 21. Common manifestations include reduced Glasgow Coma Scale (GCS) score, confusion, and focal deficit.
Management of vasospasm combines triple-H therapy (hypervolemia, hypertension, and hemodilution) with the calcium channel antagonist nimodipine20 and transcranial Doppler ultrasonography. Hypertension is achieved with inotropes and is facilitated by early occlusion of the aneurysm. Several studies have shown the benefit of volume expansion in reversing deficits,21 but triple-H therapy has yet to be tested in a randomized controlled trial. In total, 11 trials of calcium channel antagonists have been reported, and the evidence suggests that they reduce the risk of vasospasm, although the results depended mainly on one trial with nimodipine.22 The exact mechanism of action of nimodipine remains unknown, but it is now standard therapy for vasospasm prophylaxis. Detection and monitoring of vasospasm are also possible with measurement of transcranial Doppler velocities, but this technique is unreliable: It is not “on-line,” and interoperator variability is large.
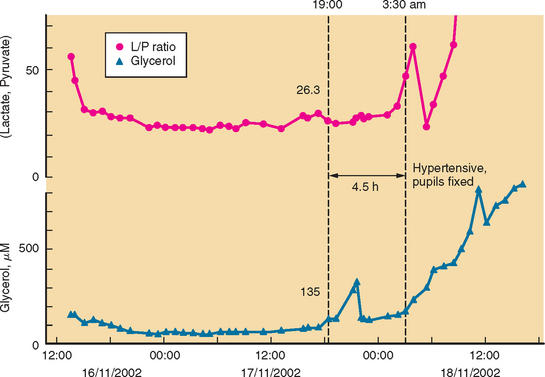
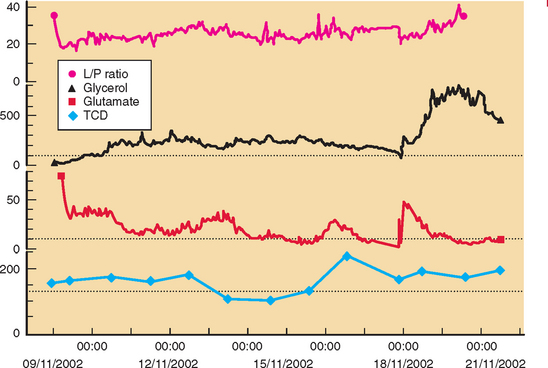
Developments such as the application of microdialysis to the injured human brain may improve monitoring and detection of cerebral vasospasm.23 There is good evidence that an increase in ratio of lactate to pyruvate precedes onset of vasospasm by several hours.24 Other studies point to the possibility that outcome after SAH is genetically predetermined.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree