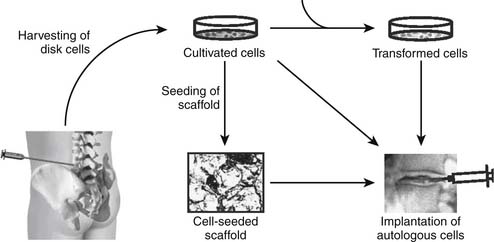
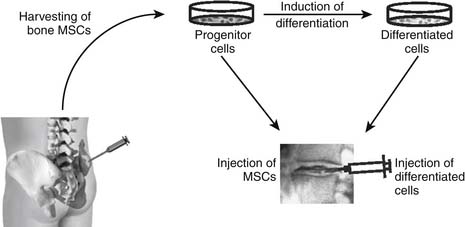
63 Intradiscal Biologics
A Potential Minimally Invasive Cure for Symptomatic Degenerative Disc Disease?
KEY POINTS
Introduction
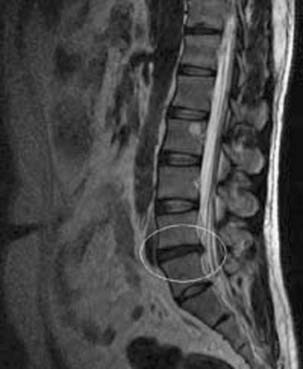
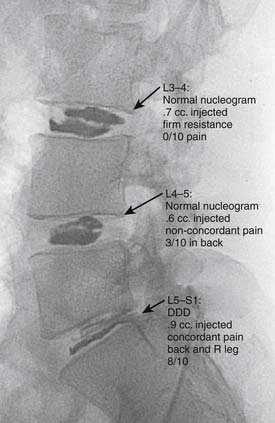
The development of a cell-based, biological replacement to restore, maintain, and improve the function of damaged tissues and organs has become the en vogue frontier to developing potential novel approaches to patient cures. The intervertebral disc (IVD) undergoes very extensive degenerative changes (Figure 63-1) with the various macro- and microtraumas that come with age and daily life activities. Individual differences have been demonstrated in young individuals exhibiting the disc of an elderly person and vice versa. It is generally accepted that an extremely prevalent rate and degree of asymptomatic disc degeneration exists in the general population. Therefore, differentiating normal aging from symptomatic pathological degeneration is very difficult and cannot be assessed by simply identifying the most abnormal disc on imaging (Figure 63-2). At this time, controlled provocative lumbar discography (Figure 63-3) remains our best clinical test to identify a physiologically painful structurally compromised IVD. The term ‘‘discogenic low back pain” is the term often used to indicate degenerative disc disease associated with concordant pain.
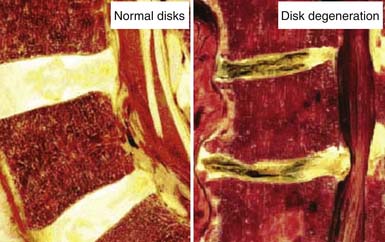
FIGURE 63-1 Macroscopic pathoanatomy evident in disc degeneration compared side by side to healthy intervertebral discs.
Functional Anatomy of the Intervertebral Disc
It is also important to note that the intervertebral disc is a largely avascular structure. With increasing age, as growth and skeletal maturation proceed, degenerative processes begin to change the morphology and therefore the function of the disc. The most widely accepted conceptual model of spinal segmental degeneration was proposed by Kirkaldy-Willis.1 In this model, the nucleus pulposus of degenerated discs is characterized by a decreased water and proteoglycan content leading to the loss of its gel-like appearance and hydrostatic properties. Degenerative changes of the annulus fibrosus are less obvious, but result in irregular lamellae with the collagen and elastin networks becoming more disorganized. Replacing the gel-like structure of the nucleus pulposus with fibrocartilaginous tissue results in decreased flexibility and therefore often in cleft formation with fissures. Up to 50% of the cells show signs of necrosis and some of them reveal signs of apoptosis, potentially resulting in cell loss from the disc.2 Although there is broad consensus about these hallmarks of degeneration, the question of whether revascularization and/or reinnervation of the inner parts of the disc may occur during degeneration is still a topic of debate.3 Although studies have described revascularization, possibly accompanied by reinnervation, of the inner parts of the IVD, it is not completely clear at which stage of degeneration these occur.3 Clarification of this question is of special importance since the interplay between neovascularization and neoinnervation might be of crucial importance for the pain sensation caused by degenerated discs. Answers to these questions may ultimately determine the rate-limiting step to the potential of biologic cures of symptomatic degenerative disc disease.
Causes of Degenerative Disc Disease
Degenerative disc disease is a complex process with a multifactorial etiology. Nutritional effects, mechanical load, and genetics all likely have contributory pathologic effects on the IVD. Of these, nutrition and removal of waste products likely play a special role in realizing the potential that intradiscal biologics may ultimately hold. Insufficient nutritional supply of the cells is thought to be the major obstacle contributing to degenerative disc disease. Cells of the IVD face the precarious situation of having to maintain a huge extracellular matrix with a ‘’fragile’’ supply of nutrients that is easily disturbed because the IVD is avascular and nutrition is dependent on diffusion. Because of the size of the intervertebral disc, the nutrients need to diffuse from a capillary network in the vertebral bodies, through the endplates and the disc matrix to the cells in the nucleus of the disc. The supply of nutrition becomes more restricted as the originally cartilaginous endplates become calcified as the degenerative process progresses. As glucose and oxygen is restricted because of diffusion distances, the removal of metabolic waste such as lactic acid becomes critically impaired. Measurements have demonstrated that as oxygen concentrations were very low in the nucleus and increased toward the disc surface, the lactic acid concentration showed the reverse profile. The buildup of lactic acid results in an intradiscal environment with a lowered pH. Low oxygen concentrations and acidic pH adversely affect the synthetic activity and proteoglycan synthesis rates of disc cells. This toxic environment may lead to a fall in proteoglycan content and therefore to degenerative disc disease. This suboptimal environment may lead to increased cell death and therefore reduced cell numbers in the disc.4 Ultimately, the result of poor nutritional supply of the IVD is that very few remaining cells are confronted with the task of maintaining an extensive matrix. Unfortunately, it likely holds true that the progression of matrix degeneration becomes irreversible once the cell density falls below a minimal threshold.
Therapeutic Biologic Strategies
Intradiscal Injection of a “Naked” Biologically Active Factor
Percutaneous intradiscal injection would provide the most straightforward approach to delivery of an active biologic factor to the disc cells (see Figure 63-3). Although direct application of potentially beneficial factors, mostly proteins like growth factors, cytokines, or anabolic enzymes, has been used frequently in vitro, few studies have been published attempting this approach in vivo. Promising results have been reported after injecting rabbit lumbar IVD in vivo with osteogenic protein-1 (OP-1), a growth factor belonging to the Transforming Growth Factor Beta (TGF-b) superfamily of growth factors. Direct injection of this growth factor resulted in significantly increased proteoglycan synthesis and restoration of disc height that was found to be stable up to 24 weeks after injection.5,6 Additional studies demonstrated that OP-1 injection exhibited a physiologic effect by inhibiting pain-related behavior in a rat disc degeneration model.7,8 Subsequently, Chubinskaya and colleagues9 documented the anticatabolic effect of intradiscal injection of OP-1 in a rat model by demonstrating reduced immunostaining for aggrecanase, Matrix Metalloproteinase (MMP)-13, substance P, Tumor necrosis factor (TNF)-α, and Interleukin (IL)-1β. Because substance P is a neuropeptide linked with inflammation and pain, the aforementioned reduction in level of this noxious protein support the previously stated physiologic inhibition of pain-related behavior.7–9 Furthermore, Miyamoto and coworkers10 were able to demonstrate that intradiscal injection of OP-1 restored the biomechanical properties of IVDs in the rabbit model of degenerative disc disease. They reported not only that a single injection of OP-1 significantly restored IVD height, but also that the treated discs demonstrated a higher viscous and elastic modulus due to increased proteoglycan content in the nucleus as well as increased collagen content in the nucleus and annulus. Concerns regarding the potential of ectopic bone formation in the epidural space with OP-1 therapies was addressed by Kawakami and associates.11 They demonstrated that there was no macroscopic evidence of ectopic bone formation, no motor paresis, and no behavioral differences to motor stimuli with epidural administration of OP-1 in a rat model. The aforementioned studies demonstrate the feasibility of direct injection, yet this technique may be limited to the presence of disc cells that are still healthy, numerous, and able to respond to a biologically active agent. Taking into context the decreasing viability and synthetic activity of human disc cells during progressive degeneration, future directions for this technique may be best suited for success in the younger patient population with discogenic Low back pain (LBP) due to modestly degenerated discs in synergistic combinations with other biologically active agents.
As opposed to injecting a biologically active enzyme or growth factor, Klein and colleagues12 published a clinical pilot study utilizing direct injection of a mixture of matrix components and aiding components known to induce proteoglycan synthesis. This solution of glucosamine and chondroitin sulfate combined with hypertonic dextrose and dimethylsulfoxide was injected into 30 patients who exhibited concordant pain on provocative lumbar discography. These patients responded well regarding reduction in a disability score and a visual analogue pain score at an average follow-up of 13 months. The authors suggested that the good outcome might be due to the combination of several components resulting in a parallel replenishing of the matrix by increased proteoglycan synthesis and induction of disc repair by simultaneous induction of multiple growth factors. This approach might prove superior to the injection of a single bioactive factor. It is conceivable that the injected matrix components are able to modulate and improve the intradiscal environment, enabling the disc cells, even in a degenerated disc, to react to the resulting secretion of growth factors and continue with the maintenance of the cellular environment. However, from this pilot study it is not clear if the injected components will be contained inside the disc in heavily degenerated discs and therefore be able to ensure a prolonged beneficial effect on the disc cells. Further controlled comparative studies are required before any therapeutic conclusions can be rendered.
Common to these injection techniques is the concern that the aforementioned demonstrated short-term effects might cease when the originally injected material has been consumed or is lost to the disc cells by diffusion. In order to provide the disc with a continuing supply of biologically active factors, it would be desirable to continuously produce the biologic of choice or include the substance in a pharmacological slow-release system as suggested for the use of growth factors.13 Considering these data, it is conceivable that combination therapy of growth factor(s) and matrix replenishment might be the way to obtain a more sustained improvement of degenerated discs. This approach may also allow for expansion of the previously stated limitations with application of this technique to various grades of degeneration because of the requirement of a certain density of healthy disc cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree