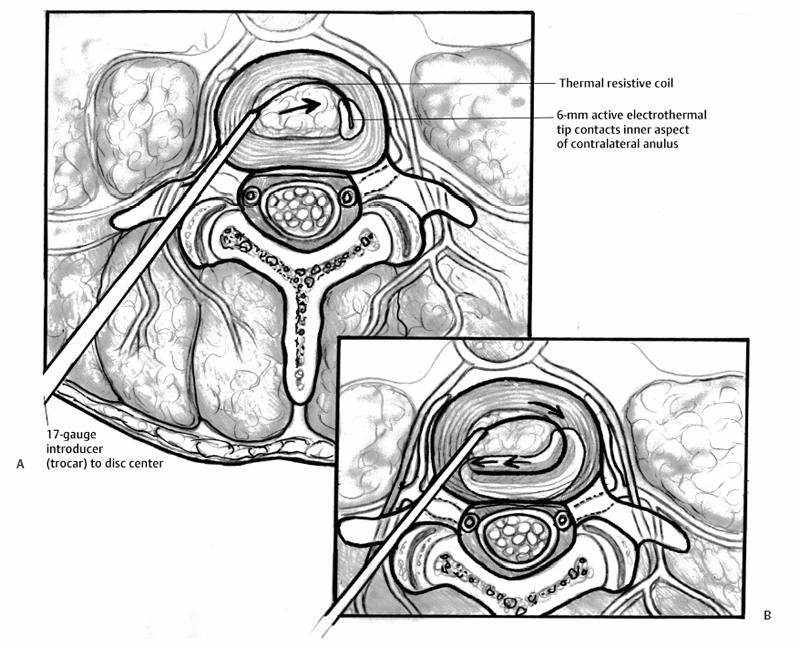
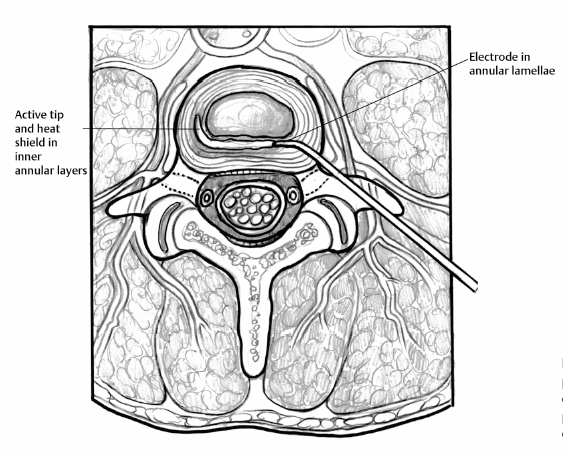
21 Intradiscal Therapy Steven Helper and Curtis W. Slipman The term “internal disc disruption” syndrome (IDDS) was coined by Crock1,2 to identify the syndrome of low back pain (LBP) and nonradicular referred pain in the setting of degenerative disc disease; and has evolved to encompass the entity marked by radial and circumferential tears in the anulus fibrosus (AF) associated with back greater than leg pain, radiation in a somatic referral pattern, and no focal neurologic deficit. Treatment has traditionally been limited to either conservative medical management or surgical fusion. Surgical treatment of these patients has yielded mixed results.3–12 Given the prevalence of this problem and the limited treatment options, the development of alternative treatment methods is the logical advancement of care. The recognition of the obvious disparity within the existing treatment paradigm has led to the evolution of minimally invasive, fluoroscopically guided, intradiscal procedures as another step in the treatment algorithm for chronic discogenic pain. These intradiscal methods include intradiscal electrothermal therapy (IDET), radiofrequency (RF) posterior ablation, intradiscal radiofrequency, intradiscal steroid instillation, percutaneous laser disc decompression (PLDD), and nucleoplasty (coblation). Despite early promising results, no single approach has proven itself to be the definitive minimally invasive solution to internal disc disruption. Recently, the use of heat therapy for controlled contraction, or shrinkage, of collagenous tissues has been evaluated. The prominent modes of thermal energy used in surgical applications are laser and RF.13–21 The advantage of RF thermal energy is its ability to precisely target tissue, while simultaneously being accurately measured with temperature control technology. Letcher and Goldring22 demonstrated that RF current and heat preferentially block smaller C fibers before the larger A-group, raising the possibility of using heat to modify nerves that transmit pain. It has been demonstrated that temperatures in and above the range of 42° to 50°C are cytotoxic to nerve fibers.23–25 This preference for small unmyelinated pain fibers, in combination with the accurate control over the location of the lesion, theoretically makes RF precise for treating various painful spinal ailments.26–31 The antinociceptive effect of percutaneous intradiscal radiofrequency thermocoagulation (PIRFT) is hypothesized to be due to a temperature increase and subsequent ablation of free nerve endings in the outer AF. Patient positioning is identical to that of provocative discography.32 A 20-gauge C15 cannula with a 10-mm exposed tip is introduced under fluoroscopic guidance. With the tip of the cannula in the center of the disc, the stylet of the cannula is replaced by the RF probe (Integra Radionics, Burlington, MA). Electrical stimulation is then performed to ensure that the electrode is not positioned near nerve structures. Stimulation at 50 and 2 Hz is used to rule out sensory and motor activation, respectively. A 90-second 70°C lesion is then made. Typically, this lesion is painless and no local anesthetic is required. Temperature monitoring is maintained throughout the procedure. In their 1996 pilot study, Van Kleef et al33 reported a 70% success rate, but the follow-up evaluation interval was only 8 weeks. In a subsequent prospective double-blind randomized trial of 28 patients, Barendse et al30 failed to demonstrate a clinical effect of PIRFT for reducing pain, functional disability, and physical impairment in patients with chronic discogenic LBP. In this trial, patients with a history of at least 1 year of chronic LBP were selected based on a diagnostic analgesic discography. Twenty-eight patients with a single painful disc were selected and randomly assigned to receive PIRFT or a sham procedure. At 8 weeks after treatment there was one success (1 of 13 patients) in the RF group and two (2 of 15 patients) in the control group. Ercelen et al34 conducted a randomized trial of RF lesioning using two different time modalities. In their study, 60 patients with chronic LBP were selected for provocative discography after failing 2 years of conservative management. Thirty-nine patients (39 of 60 patients) with positive discography results were randomly selected and divided into two groups. In the first group, treatment was performed for 120 seconds, and in the second group for 360 seconds, both at 80°C. Although the immediate, 1-week, 2-week, and 1-month Visual Analog Scale (VAS) scores were decreased significantly in both groups, no statistical differences were found for pain relief and functional improvement between the two groups. At 6 months, the VAS scores returned to baseline values demonstrating the absence of a sustained beneficial effect. Houpt et al35 challenged the validity of thermocoagulation of annular nociceptors with PIRFT as a means of treating discogenic pain. They measured temperature changes within the human IVD during transient intranuclear heating demonstrating that temperature changes at distances further than 11 mm were insufficient to raise the tissue temperature to that needed for neuronal cell death. The authors appropriately concluded the possible clinical effects of RF heating of IVDs are not due to thermal denervation of the disc. Overall, there remains no evidence to support the use of PIRFT in the management of lumbar internal disc disruption syndrome. IDET is an intradiscal heating technique that is founded on the notion that the IVD itself is an avascular structure. If an external heating mechanism such as IDET is used to elevate temperatures within the disc there tends to be minimal fluctuation due to the absence of a circulatory system buffer. Heating external to the disc is quickly dissipated by the “heat sink” created by the vascular and cerebrospinal fluid circulation outside the disc, thereby protecting adjacent structures from injury.36,37 IDET transfers heat by conduction from a thermal resistive coil to the adjacent tissue. Temperature sensors deliver feedback to the generator, which adjusts power levels as necessary to reach and maintain set target temperatures. Using a standard discographic approach, a 17-gauge introducer is placed into the center of the disc (Fig. 21.1). A navigable intradiscal catheter with a 6-cm active electrothermal tip (SpineCATH; Smith & Nephew Inc., Andover, MA) is then advanced through the trocar to pass completely across the nucleus pulposus until it contacts the inner aspect of the contralateral anterolateral anulus. With continued insertion, the electrode deflects circumferentially back, along the inner perimeter of the anulus, toward the insertion side. The catheter is then attached to the RF generator and the disc is gradually heated to a temperature of 90°C over 12.5 minutes. This peak temperature is maintained for 4 more minutes. Postprocedure, a lumbar support brace is worn for 6 weeks to deter movements that might elevate intradiscal pressure (i.e., forward flexion). IDET was developed to address the failure of PIRFT to reach therapeutic temperatures at the target tissues.35–37 The proposed mechanism is a combination of annular collagen shrinkage, stabilizing annular fissures, and thermocoagulation of native nociceptors and ingrown unmyelinated nerve fibers. Initial data on IDET, in cadaveric specimens and live human subjects, showed an average maximum temperature at the outer anulus of 44.8°C and 47.5°C, respectively.36,37 Similarly, Shah et al demonstrated outer annular temperatures ~55°C, seemingly adequate to provide neural blockade.38 Freeman et al followed suit in their detailed in vivo study on sheep disc. Again, thermal mapping seemed to demonstrate adequate heating. However, postoperative histology failed to substantiate denervation of nerve fibers in the outer lamellae of the anulus. Kleinstueck et al40 gave a plausible explanation of this paradox. By their calculations, with the use of the existing protocol for the IDET procedure,13,17,44 destruction of neural tissue is limited to within 6 mm of the IDET heating probe. According to Kleinstueck et al’s data, neurotoxic temperatures were found in the outer annular fibers only 55% of the time.40 Bono et al44a then demonstrated comparable although slightly higher temperatures at varying distances from the heating coil. In fact, more than 45°C was achieved in 71% of specimens at distances of 9 to 14 mm outside the coil. It is evident from the data of either study that not all discs will achieve sufficient temperatures for nociceptive denervation in clinically relevant regions of the disc. Perhaps this is one explanation for the modest clinical efficacy demonstrated in published trials.13,14,17–19,21,44–46 Several studies have examined the histologic effects of targeted thermal therapy on collagen.47–61 Hecht et al47 and Naseef et al49 have studied the effects of RF effects on collagen tissue. Significant ultrastructural alterations in collagenous architecture occur during capsular heating. Thermal coagulation or coagulation necrosis will occur in collagen exposed to temperatures greater than ~60° to 65°C for a duration of minutes. 47,50,51,62,63 In Shah et al’s histologic study,38 outer annular temperatures reached 55°C, which should not be sufficient to cause collagen shrinkage. Nevertheless, electron microscopy revealed shrinkage and clustering of collagen fibrils, annular disorganization, and cellular debris. Kleinstueck et al40 also failed to demonstrate temperatures in excess of 60°C in the outer annular fibers. Paradoxically, despite posterior annular temperatures exceeding 60°C in 28 of 40 levels (70%) on thermal mapping, Freeman et al39 did not show a significant histologic effect on experimentally induced annular tears. Scientific inquiries to date suggest that the reported benefit from IDET appears to be related to factors other than annular collagen shrinkage or the stabilization of annular fissures. Even if the proposed theories of nociceptor ablation and collagen denaturation are valid, there remain variations of disc pathology and anatomy between patients and uncertainty regarding the exact pain source location.64,65 Fig. 21.1 (A) Intradiscal electrothermal annuloplasty. Cross-sectional image of an intradiscal electrothermal annuloplasty catheter as it is coiled posteriorly along the inner annular fibers of the intervertebral disc. (B) Intradiscal electrothermal annuloplasty. Cross-sectional image of an intradiscal electrothermal annuloplasty catheter properly coiled within the lumbar disc. Alternate theories to explain the modest efficacy of IDET do exist. One possibility is that the procedure may denervate nerve endings in the adjacent endplate. Another relates to the prevention of leaching of chemical irritants through injured endplates or annular tears. Injured or herniated discs are known to produce chemical mediators such as phospholipase A2, nitric oxide, and metalloproteinase, which are associated with inflammation and repair. It is conceivable that IDET may reduce leakage of these chemical irritants through the annular tear so that periannular nociceptors are not stimulated. In 1998, Saal and Saal36 reported the preliminary results for 25 consecutive patients treated with IDET. Each patient underwent lumbar discography following failed conservative care for axial LBP. At a mean follow-up period of 7 months, 20 of 25 patients (80%) reported a 2-point reduction in their VAS scores. A successful functional outcome as measured by the SF-36 Health Survey was achieved in 77% of the patients treated at a single level, 75% of the patients treated at two or more levels. In 2000, Saal and Saal44 completed and published the first prospective case series on the success of IDET. Following a screening of 1116 consecutive patients, a cohort of 62 patients (0.6%) met the following inclusion criteria: at least 6 months of nonsurgical treatment; a normal neurologic exam; negative straight leg raise on exam; magnetic resonance imaging negative for neural compromise; and concordant pain on discography. Thirty underwent single-level IDET and 32 underwent multilevel treatment. The mean change between baseline and follow-up VAS score was 3.0 (p < 0.001), mean change in SF-36 physical functioning (SF-36-PF) score was 20 (p < 0.001), and mean change in SF-36 bodily pain (SF-36-BP) score was 17 (p < 0.001). Symptomatic improvement of SF-36-PF, SF-36-BP, and VAS was 71%, 74%, and 71%, respectively. Less favorable outcomes were observed in those patients with decreased disc height of at least 30%. The same authors later reported their 2-year outcomes on 58 of the original 62 patients.13 The mean change between baseline and follow-up VAS score was 3.2, mean change in SF-36-PF was 31, and mean change in SF-36-BP was 22. Seventy-two percent of patients experienced at least a 2-point reduction on VAS, and 50% noted at least a 4-point reduction in pain. Symptomatic improvement of SF-36-PF, SF-36-BP, and VAS was 82%, 78%, and 72%, respectively. No significant statistical differences were noted between one-level and two-level IDET cases or between private pay patients and worker’s compensation. In 2000, Karasek and Bogduk published the first IDET prospective case-control outcome study.14 Fifty-two patients with symptoms consistent with IDDS and positive provocation discograms, were studied. Thirty-five (35 of 52) of these patients were treated with IDET and were prospectively compared with 17 controls. This was not a randomized trial, as the control group was composed of patients who could not undergo IDET because their insurance company denied payment. Data were obtained before treatment and at 3, 6, and 12 months after treatment. At 1 year, 60% (95% CI ± 16%) of the IDET-treated group reported a satisfactory drop in their VAS pain scores. Only 23% (95% CI ± 14%) described complete pain relief. All but one patient (16 of 17 patients) within the control group continued to have persistent LBP. Interestingly, the lack of improvement seen in the control group seems to be worse than the rates of improvement by natural history alone.15 Whether or not the natural history of IDDS is clearly known, the poor outcomes in the control group in Karasek and Bogduk’s 2000 study14 are subject to negative influence by patient expectations.16 In 2002, the same authors17 reported on their 2-year outcomes when compared with the 1-year data. Improvements observed at 1 year persisted in the IDET and were significantly different from the comparison group (p < 0.001). Fifty-four percent of the IDET-treated group reported had continued pain relief of ≥ 50% (18 of 35 patients). Derby et al17,18 reported favorable outcomes in 62.5% of their IDET-treated cohort at 6 months. The mean VAS reduction was 1.84 (standard deviation [SD] 2.38), which may not be clinically significant. They also reported that 25% of IDET-treated patients did not appreciate any benefit at 12 months, and 12.5% of patients had a worsening of three out of four outcome scales. In 2002, Gerszten et al19 reported their results of a nonrandomized, prospective case series of 27 patients with 1-year follow-up. Seventy-five percent of patients noted improvement on the Roland-Morris Disability Questionnaire (RMDQ), yet only 47% improved on the SF-36 survey. As reported by Bogduk and Karasek,14,17 no relationship was found between outcome and symptom duration (p = 0.32), number of levels treated (p = 0.20), and worker’s compensation status (p = 0.38). In 2003, Lutz et al63a published the outcomes of their nonrandomized, prospective case series of 33 patients diagnosed with internal disc disruption by provocation discography. There was a mean follow-up of 15 months. A mean improvement of 3.9 points on the VAS (p < 0.001), a mean change in the lower limb VAS score of 3.7 (p < 0.001), and a mean change in the RMDQ of 7.3 (p < 0.001) was reported. Complete pain relief was achieved in only 24% of the patients and partial pain relief was noted in just 46% of the patients. No statistically significant difference was found between the demographics of responders versus nonresponders, single-level versus multilevel IDET outcomes, patients with low pressure (concordant pain at <15 psi above baseline opening pressure) versus high pressure (15 psi to 50 psi above baseline opening pressure) sensitive discs, and patients with workers’ compensation versus no-fault cases. In 2003, a bi-institutional, retrospective chart review study was published by Cohen et al20 to determine complication rates and risk factors for IDET failure. Notwithstanding the title, the primary outcome measure for this study was pain relief. Over 50% reduction in pain 6 months postprocedure was defined as success and any other result was labeled as failure. Overall, 48% (38 of 79) of patients reported more than 50% pain relief persisting at their 6-month follow-up, with eight of these patients (10%) obtaining over 90% relief. To help explain their modest success rate, the authors draw attention to their liberal inclusion criteria. The first double-blind, randomized, controlled trial for IDET was published in 2004. Pauza et al21 identified 1360 potential subjects willing to submit to randomization and narrowed this number to 260 patients after clinical examination. Of these, only 64 proved discogram-positive and were randomized into the IDET treatment group (n = 37) or the matched sham control group (n = 27). Although both groups demonstrated mean improvements in pain, disability, and depression, the improvements were better in the IDET-treated group. In the IDET group, a mere 13.5% of treated patients had total pain relief, and only 40% had a partial response. That means 50% of the treated patients appreciated no significant benefit from IDET, which is in stark contrast to the 25% reported in the uncontrolled trial by Derby et al.18 Thus, it seems that IDET may have a modest effect over placebo in only a limited percentage of well-selected individuals. Freeman et al45 followed with their own randomized, double-blind, controlled trial.45 Inclusion criteria included the presence of one- or two-level symptomatic disc degeneration with posterior or posterolateral annular tears as determined by provocative computed tomography discography. Fifty-seven patients were randomized with a 2:1 ratio: 38 to IDET and 19 to a sham procedure. Similar to Pauza et al,21 the number of subjects included in each arm of the study was less than desired to achieve a power of 80%.45 A comprehensive set of outcome measures was recorded at baseline and 6 months. There was no significant change in outcome measures in either group at 6 months. Freeman et al45 concluded that IDET is no more effective than placebo for the treatment of chronic discogenic pain. The conclusions by Freeman et al45 are quite different than those of Pauza et al.21 One study examined the use of IDET in a particular population.66 Freedman et al66 reported on the results of 36 active-duty soldiers (34 men, 2 women) who underwent IDET for chronic discogenic LBP unresponsive to conservative therapy. Data were collected through clinic chart review and follow-up questionnaires. Success was defined as a 50% decrease in pain from baseline. At 6 months, the success rate was 47% (17 of 36). These numbers deteriorated to 16% (5 of 31 patients) at final follow-up (average 29.7 months). Almost 20% of patients felt worse at their most recent follow-up. Seven of 31 soldiers (23%), all men, went on to spinal surgery within 24 months of failed IDET. The authors concluded IDET was not an adequate alternative to spinal fusion for treatment of chronic discogenic LBP in active-duty soldiers. One retrospective study that must be considered when attempting to determine the efficacy of IDET is the 1-year data by Davis et al.46 They reported that 97% of post-IDET patients continued to have functionally limiting LBP, 29% reported having more LBP than before the IDET procedure, 29% required more narcotics than pre-IDET, and 53% of the patients were dissatisfied with their outcome. Interestingly, when queried as to whether they would undergo the procedure again, 53% said they would and 31% said they would not. The substantial percentage of patients willing to undergo a second IDET procedure, despite poor results as measured by the other outcome measures, may be related to the general preference of a patient to undergo a minimally invasive treatment before considering surgery. Radiofrequency posterior annuloplasty (RFA) is also commonly known by the name of the device used to create the lesion, the DiscTRODE (Valleylab, Tyco HealthCare Group). Unlike the SpineCATH used with IDET, this flexible radiofrequency electrode can be directly placed in the posterior and posterolateral mid-anulus (Fig. 21.2). The electrode is inserted on the contralateral side to the annular tear and is navigated directly across the posterior anulus, between the annular lamellae. The depth of insertion is controlled by use of electrical impedance values and radiologic positioning. Once the electrode is placed, the anulus is heated so that the electrode registers incremental temperatures of 55° to 65°C over 14 minutes.
Intradiscal Heating Procedures
Percutaneous Intradiscal Radiofrequency Thermocoagulation
Procedure
Clinical Outcomes
Intradiscal Electrothermal Therapy
Procedure
Mechanism of Action
Effect on Nerves
Effect on Collagen
Clinical Outcomes
Radiofrequency Posterior Annuloplasty
Procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








