Chapter 102 Intradural Extramedullary Spinal Lesions
Before the advent of microsurgical techniques, surgery of many spinal cord neoplasms consisted primarily of open biopsy and radiation therapy.1–3 Recent technologic advances in neurosurgery and diagnostic imaging have expanded the role for operative treatment of spinal tumors. Although Horsely performed the first successful excision of a spinal tumor in 1887, and Elsberg and Frazier advocated resection of spinal tumors in the early part of the 20th century, consistently acceptable morbidity and mortality were not realized until recently.4–7 MRI has facilitated preoperative localization and surgical planning. The use of intraoperative neurologic monitoring and ultrasound has led to reduced operative morbidity. Advances in microsurgical techniques as well as the development of ultrasonic aspiration and laser technology have established microsurgical removal as the most effective treatment for benign intradural extramedullary tumors.
Incidence and Pathology
Tumors of the spine are anatomically classified by their relationship to the dura mater and spinal cord parenchyma. Intradural tumors can be intramedullary or extramedullary, and extramedullary tumors account for approximately three fourths of all intradural spinal tumors.1,5,8,9 Intradural spinal neoplasms make up approximately 10% of primary central nervous system tumors in adults,1,10 and about two thirds are extramedullary, histologically benign, and well circumscribed. Meningioma, schwannoma, and filum terminale ependymoma are the most common histopathologic lesions in the intradural extramedullary space. Meningiomas and nerve sheath neoplasms account for 80% of extramedullary spinal cord tumors, and filum terminale ependymomas make up 15% of these lesions. The remaining 5% includes paragangliomas, drop metastases, and granulomas, all of which are rare.
Meningiomas
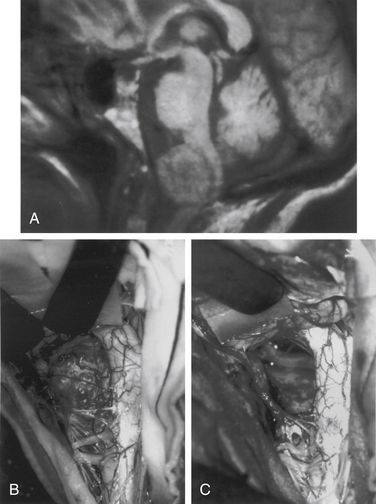
Meningiomas arise from arachnoid cap cells embedded in the dura mater near the nerve root sleeve, reflecting their predominant lateral location and meningeal attachment. Other possible cells of origin include fibroblasts associated with the dura or pia mater, which may account for the occasional ventral or dorsal location of these tumors. Meningiomas occur in all age-groups, but most arise in people between the fifth and seventh decades of life. Women account for 75% to 85% of cases, and about 80% of tumors are thoracic.11–13 The upper cervical spine and foramen magnum are also common sites14 (Fig. 102-1). Here, meningiomas often occupy a ventral or ventrolateral position and may adhere to the vertebral artery near its intradural entry and initial intracranial course. Low cervical and lumbar meningiomas are infrequent. Most spinal meningiomas are entirely intradural; however, about 10% can be both intradural and extradural or entirely extradural.12 Meningiomas are generally solitary, but multiplicity can be observed in patients with neurofibromatosis. The overall incidence of multiplicity in the spine is 1% to 2%.15
Nerve Sheath Tumors: Schwannomas and Neurofibromas
Nerve sheath tumors are categorized as either schwannomas or neurofibromas. Although evidence from tissue culture, electron microscopy, and immunohistochemistry supports a common Schwann cell origin for neurofibromas and schwannomas, the morphologic heterogeneity of neurofibromas suggests participation of additional cell types such as perineural cells and fibroblasts. Neurofibromas and schwannomas merit separate consideration because of distinct demographic, histologic, and biologic characteristics. A schwannoma appears grossly as a smooth, globoid mass that does not produce enlargement of the nerve but is suspended eccentrically from it, sometimes by a discrete attachment. The histologic appearance consists of elongated bipolar cells with fusiform, darkly staining nuclei arranged in compact interlacing fascicles that tend to palisade (Antoni A pattern). A loosely arranged pattern of stellate cells (Antoni B pattern) is less common.10 The histologic appearance of a neurofibroma consists of an abundance of fibrous tissue and the conspicuous presence of nerve fibers within the tumor stroma.16 Grossly, the tumor produces fusiform enlargement of the involved nerve, which makes it impossible to distinguish between them. Multiple neurofibromas establish the diagnosis of neurofibromatosis, but this syndrome should be considered even in patients with solitary involvement.
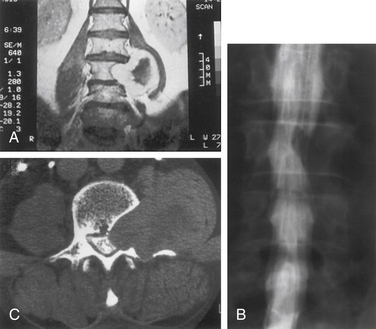
Nerve sheath tumors account for about 25% of intradural spinal cord tumors in adults.12,17 Most are solitary schwannomas occurring throughout the spinal canal. The fourth through sixth decades of life represent the peak incidence of occurrence, and men and women are equally affected. Most nerve sheath tumors arise from a dorsal nerve root. Ventral root tumors are more commonly neurofibromas. Most nerve sheath tumors are entirely intradural, but in 10% to 15% of cases they extend through the dural root sleeve as a dumb-bell–shaped tumor with both intradural and extradural components12 (Fig. 102-2). About 10% of nerve sheath tumors are epidural or paraspinal in location. Intramedullary nerve sheath tumors account for only 1% and are believed to arise from the perivascular nerve sheaths that accompany penetrating spinal cord vessels. Centripetal growth of a nerve sheath tumor can also result in subpial extension, and this occurs most often with plexiform neurofibromas. In these cases, both intramedullary and extramedullary tumor components are apparent. Brachial or lumbar plexus neurofibromas can extend centrally into the intradural space along multiple nerve roots. Conversely, retrograde intraspinal extension of a paraspinal schwannoma usually remains epidural.
About 2.5% of intradural spinal nerve sheath tumors are malignant,18 and at least one half of these occur in patients with neurofibromatosis. Rarely, in children, malignant nerve sheath tumors can be widely disseminated.19 These tumors carry a poor prognosis, and survival is generally less than 1 year. These tumors must be distinguished from the rare cellular schwannoma, which has aggressive histologic features but is associated with a favorable prognosis. Occasionally, malignant melanoma can involve spinal nerve roots and radiographically mimic a neurofibroma.20
Filum Terminale Ependymomas
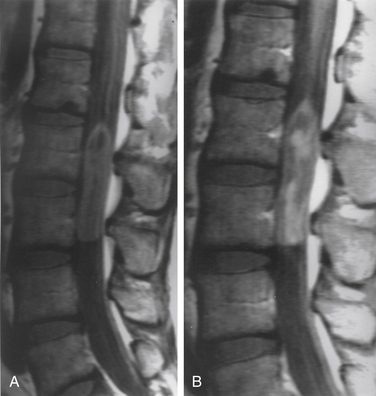
Although filum ependymomas have been classified as intramedullary lesions by virtue of the neuroectodermal derivation of the filum terminale, it is appropriate to consider them with extramedullary tumors from an anatomic and surgical perspective.1,7 About 40% of spinal canal ependymomas arise within the filum terminale1 (Fig. 102-3), most occurring in its proximal intradural portion. Astrocytomas, oligodendrogliomas, and paragangliomas can also originate in the filum but are rare. Filum terminale ependymomas occur throughout life but are most common in the third to fifth decades. Men are slightly more commonly affected. Filum ependymomas and cauda equina nerve sheath tumors occur with about equal frequency in men and women.21,22
Lesions are typically reddish, sausage-shaped growths with moderate vascularity. Although unencapsulated, they are usually well circumscribed and may be covered by arachnoid. They can present with widespread disease, and lesions in the lumbar spine may represent drop metastases from the posterior fossa or other sites. Myxopapillary ependymoma is the most common histologic type encountered. The microscopic appearance consists of a papillary arrangement of cuboidal or columnar tumor cells surrounding a vascularized core of hyalinized and hypocellular connective tissue.10 Nearly all are histologically benign.23 These tumors, however, tend to be more aggressive in people in younger age groups.24
Miscellaneous Pathology
Extramedullary masses can be neoplastic or non-neoplastic. Paragangliomas are rare tumors of neural crest origin arising from the filum terminale or cauda equina.25 They are benign and usually nonfunctioning tumors that histologically resemble extra-adrenal paraganglia. They appear grossly as well-circumscribed vascular tumors and may be clinically and radiographically indistinguishable from filum terminale ependymomas. Identification of dense-core neurosecretory granules on electron microscopy establishes the diagnosis, and complete removal can be accomplished in most cases. Cavernous malformations, hemangioblastomas, and ganglioneuromas may involve an intradural nerve root and appear as extramedullary masses. These lesions can be observed clinically as nerve sheath tumors with early radicular symptoms. Ganglioneuromas may be manifested as dumb-bell–shaped tumors in pediatric patients.
Dermoids, epidermoids, lipomas, teratomas, and neurenteric cysts are inclusion lesions resulting from disordered embryogenesis.26,27 They can occur throughout the spinal canal but are more common in the thoracolumbar and lumbar spine. Intramedullary locations have also been reported. Associated anomalies such as cutaneous lesions, sinus tracts, occult ventral or dorsal rachischisis, and split cord malformations may be present.27,28 Inclusion tumors and cysts are generally seen as masses, but recurrent meningitis, tethered cord syndrome, or congenital deformities may be the predominant clinical finding.
Non-neoplastic lesions may also appear as extramedullary masses, arachnoid cysts being a well-known example. These cysts are most common in the thoracic spine and are usually dorsal to the spinal cord.29 Intraspinal aneurysms are extremely rare. Herniated intervertebral discs have occasionally been reported to rupture through the dura and appear as an intradural extramedullary mass.30
Inflammatory pathologies such as sarcoidosis, tuberculoma, and subdural empyema are rarely seen as intradural mass lesions.30–32 In patients with intrathecal drug delivery systems, especially morphine pumps, intradural granulomas may form around the catheter tip, causing progressive neurologic decline.33
Although spinal carcinomatous meningitis frequently complicates systemic cancer, secondary metastatic mass lesions of the intradural extramedullary compartment are rare. Malignant intracranial neoplasms that oppose the subarachnoid space or ventricles are the most likely intracranial tumors to demonstrate cerebrospinal fluid (CSF) drop metastasis into the spinal subarachnoid space.4 Systemic cancer accesses the subarachnoid space, either through direct dural root sleeve penetration or, more commonly, hematogenously via the choroid plexus.34,35
Clinical Features
Extramedullary spinal cord lesions cause a variety of clinical signs and symptoms, and no particular clinical syndrome is pathognomonic. In general, pain followed by progressive neurologic deficit is the clinical course most often encountered. The classic syndrome historically ascribed to intradural extramedullary tumors consists of progression through segmental, hemicord, and transverse cord dysfunction.36,37 This presentation, however, is rarely observed in current clinical practice and is not specific to extramedullary lesions. Generally, the clinical features of most extramedullary tumors reflect a slow-growing intraspinal mass. Specific manifestations are variable and determined mainly by tumor location. Upper cervical and foramen magnum tumors are often ventral and are frequently accompanied by suboccipital pain, distal arm weakness, and hand intrinsic muscle weakness and atrophy causing clumsiness.14 The etiology of this well-known syndrome is uncertain, but it most likely results from venous insufficiency. Increased intracranial pressure and hydrocephalus can occur rarely with an extramedullary tumor at any level but are more common with upper cervical lesions.38 The mechanism is probably related to elevation of the CSF protein and resulting impaired CSF flow and absorption. Segmental motor weakness and long-tract signs are the hallmarks of low cervical and midcervical tumors. Early signs and symptoms are typically asymmetrical, which reflects the predominantly lateral location of most intradural tumors. Brown-Séquard syndrome, characterized by ipsilateral corticospinal spinal tract and posterior column and contralateral spinothalamic tract dysfunction, is common.
Thoracic tumors frequently produce long-tract signs, and corticospinal tracts are particularly vulnerable. Initial signs of stiffness and early muscle fatigue eventually give way to spasticity. Weakness usually begins distally, particularly with dorsiflexion of the ankle and large toe. Sensory gait ataxia may result from bilateral posterior column compression with dorsal midline tumors. Bowel and bladder functions are not significantly impaired until late in the clinical course. Filum ependymomas are characterized most frequently by back pain and subsequent asymmetrical radiation to both legs. Increased pain on recumbency, an important clinical feature of extramedullary tumors, is most often associated with large cauda equina lesions. Subarachnoid hemorrhage has also been reported as a presenting feature of an extramedullary tumor.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree