Intraoperative Electrocorticography
Mark D. Holmes
Gian-Emilio Chatrian
Introduction
The method of recording electrical potentials directly from the cerebral cortex (electrocorticography [EcoG]) or from the cerebral cortex and subjacent structures was pioneered in humans by Berger, who detected these activities with electrodes placed over the dural surface in patients with skull defects15 and with a needle inserted through the cortex and underlying white matter of a patient with a distant brain tumor.16 Subsequent work continued to be aimed at validating the cortical origin of scalp-recorded electroencephalogram (EEG) rhythms and demonstrating alterations of electrocerebral activity caused by brain tumors,2,42,98,116 but direct brain recordings soon became established primarily as aids in the surgical treatment of medically refractory partial epilepsy.7,44,54,55,57,63,78,114 The initial role of intraoperative ECoG in defining sites of ictal origin has been supplanted in recent decades by advances in continuous EEG-video monitoring (both noninvasive and invasive) and by developments in neuroimaging. The role of intraoperative ECoG has evolved primarily as a tool for better understanding the likely boundaries of the epileptogenic zone and as guide to determining the extent of surgical resection. Despite the changing role of ECoG, the technique and interpretation of direct brain recordings during surgery has changed little during the last six decades. This review summarizes traditional technical and interpretive principles of intraoperative ECoG and highlights evolving concepts of this method. The original work of Jasper55 and the comprehensive reviews of Bates,13 Ajmone-Marsan and O’Connor,8 and Gloor46 are sources of additional information.
Indications
In patients afflicted by chronic, medically intractable partial seizures, the ECoG is performed during surgery (Fig. 1) following preoperative studies that have identified an epileptogenic region in the hemisphere targeted for surgery. Intraoperative ECoG is intended to (a) identify the limits of the epileptogenic zone, (b) guide the extent of resection, and (c) assess its completeness.
Identification of the Epileptogenic Zone
Determination of the site of the epileptogenic zone by intraoperative ECoG relies on several factors, including demonstrating an area of brain that (a) displays interictal epileptiform discharges, (b) shows susceptibility to repetitive electrical stimulation, and (c) gives rise to the initial, or all, manifestations of the patient’s habitual seizures when electrically stimulated. In fact, however, it is rare that, seizure onset can be determined only by ECoG.
The Interictal Spiking Area
Because intraoperative ECoGs are of relatively short duration, electrographic seizures are rarely recorded except in some particular conditions,11,46 and spontaneous epileptic activity consists almost exclusively of interictal discharges, that is, spikes, sharp waves, and their variants. For the sake of brevity, these are referred to in this chapter as “spikes.” Because of this limitation, the utility of the ECoG during epilepsy surgery depends on the following assumptions: (a) that intraoperatively recorded interictal spikes are reliable markers of the epileptogenic process and (b) that they provide dependable information on the site and extent of epileptogenic tissue to be removed to successfully control the patient’s attacks. The following observations suggest that both of these assumptions have limited validity:
It is impossible to distinguish with assurance in ECoG recordings primary epileptiform discharges arising from a given brain location from secondary discharges propagated from a distant epileptogenic site.27,55,59
The extent of the intraoperatively determined interictal spiking area can be increased or diminished by a number of anesthetic, analgesic, and other medications commonly administered during surgery and by the intrusion of drowsiness or non–rapid eye movement (REM) sleep in the waking ECoG.66,96
Localized Electrical Stimulation of the Brain
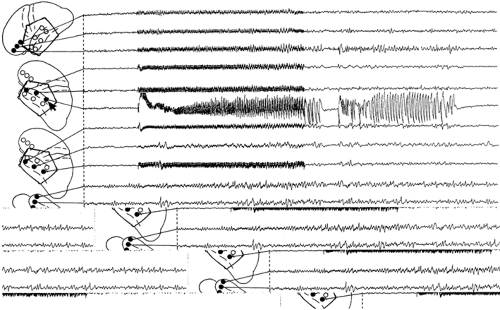
Because the topographic extent of interictal ECoG discharges provides limited assistance in defining the boundaries of the surgically relevant epileptogenic zone, attempts have been made to identify this area by localized repetitive electrical stimulation of the brain. When of sufficient intensity, this excitation elicits an “afterdischarge,” that is, a seizure discharge that typically begins at the stimulated site and subsequently either remains restricted to this location or spreads to adjacent or wider cortical regions as well as to subcortical structures (Fig. 2). Afterdischarges vary widely in duration from <1 sec to as much as 90 sec or longer and are frequently followed by postictal depression, which is in turn succeeded by slow activity of variable duration, often most obvious at the site of stimulation.55,114 Localized stimulation of the brain of patients with chronic, intractable partial seizures was originally undertaken with the hope that (a) human epileptogenic tissue might display increased susceptibility to this stimulus in the form of afterdischarges of lower threshold, longer duration, or both, relative to nonepileptogenic tissue, and (b) the afterdischarge so elicited would be accompanied by clinical changes reproducing the initial, or all, manifestations of the patient’s
habitual seizures. However, intraoperative as well as extraoperative studies have demonstrated that afterdischarge thresholds and durations vary among cortical areas55,114 and even within the same area from day to day114 and from one stimulation to the next.3 Their thresholds were elevated shortly after a prolonged afterdischarge114 and were altered by structural changes in the area stimulated, although conflicting results were obtained by stimulating normal and sclerotic human hippocampi.17,26 In addition, afterdischarges could be especially prominent and long-lasting at locations not demonstrating interictal spikes,46 were not consistently related to the site of spontaneous ictal onset,17 and sometimes occurred solely, or persisted longer, at sites distant from those stimulated.55
habitual seizures. However, intraoperative as well as extraoperative studies have demonstrated that afterdischarge thresholds and durations vary among cortical areas55,114 and even within the same area from day to day114 and from one stimulation to the next.3 Their thresholds were elevated shortly after a prolonged afterdischarge114 and were altered by structural changes in the area stimulated, although conflicting results were obtained by stimulating normal and sclerotic human hippocampi.17,26 In addition, afterdischarges could be especially prominent and long-lasting at locations not demonstrating interictal spikes,46 were not consistently related to the site of spontaneous ictal onset,17 and sometimes occurred solely, or persisted longer, at sites distant from those stimulated.55
Penfield and his associates37,46,83,94 among others made extensive use of localized electrical stimulation of the brain to reproduce in waking patients undergoing surgery the initial, or all, manifestations of their habitual partial seizures. The ability of this technique to localize the epileptogenic zone depended on the validity of the assumptions that (a) the initial manifestations of partial seizures, that is, the “auras,” indicated the site of the brain from which the seizures developed and (b) the reproduction of these manifestations by electrical stimulation of a restricted area of the brain confirmed this localization. Conflicting opinions were expressed on the localizing significance of the aura,5,82,105,114 and attempts to reproduce it intraoperatively or extraoperatively by electrical stimulation of cortical
and subcortical structures provided evidence that, in many instances, the aura did not reflect an ictal discharge arising at the site stimulated but resulted from spread of seizure activity at a distance from this location.12,34,114,120 The results of one study40 indicated that the electrically elicited initial manifestations of the patient’s habitual seizures, whether occurring in the presence of a localized afterdischarge or at the very onset of a spreading afterdischarge, lacked lateralizing or specific localizing value and at best suggested the most likely lobar origin of the patient’s spontaneous attacks. Clinical manifestations appearing during the course of a spreading afterdischarge were likely related to the involvement of brain areas other than that stimulated.
and subcortical structures provided evidence that, in many instances, the aura did not reflect an ictal discharge arising at the site stimulated but resulted from spread of seizure activity at a distance from this location.12,34,114,120 The results of one study40 indicated that the electrically elicited initial manifestations of the patient’s habitual seizures, whether occurring in the presence of a localized afterdischarge or at the very onset of a spreading afterdischarge, lacked lateralizing or specific localizing value and at best suggested the most likely lobar origin of the patient’s spontaneous attacks. Clinical manifestations appearing during the course of a spreading afterdischarge were likely related to the involvement of brain areas other than that stimulated.
The available information indicates that the topography of intraoperative interictal spiking, the afterdischarge threshold and duration, and the reproduction of the clinical manifestations of the patient’s seizures by localized electrical stimulation of the brain do not consistently identify the epileptogenic zone.
Functional Mapping
Additional assistance in determining the boundaries of “tailored” resections of epileptogenic tissue is offered by the identification in waking patients of motor and sensory cortices and the delineation of regions involved in speech and memory functions, which are achieved by localized electrical stimulation of the brain at intensities mostly subliminal for afterdischarge (see Chapter 174, Intraoperative Functional Mapping).
Technique
Electrodes and Instrumentation
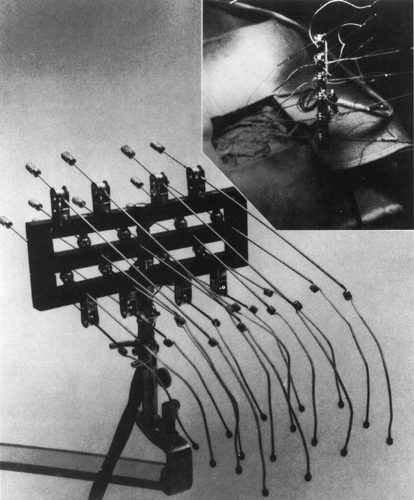
ECoG electrodes8,13 must be capable of detecting the electrical activities of the cortex exposed by craniotomy and of adjacent as well as more remote areas of the lateral, inferior, and medial surfaces and, at times, subcortical areas of the hemisphere undergoing surgery. Most commonly, electrode-holder assemblies (Fig. 1) are used that typically incorporate 16 insulated silver wires. The distal ends of these wires terminate with a carbon ball32 or a silver/silver chloride ball covered with cotton soaked in physiologic saline solution, which makes contact with the exposed cortex. Their proximal ends are connected by coiled springs to thin stainless steel rods, which are mounted on the universal ball joints of a holder clamped to the bone edge of the craniotomy. Wires from these joints lead to the inputs of the recording system. An alternative technique uses variously shaped assemblies of 4 to 64 or more small platinum, silver, or stainless steel disk electrodes embedded 10 to 15 mm apart in soft, flexible, clear, silicone plastic (Silastic) sheets.121 These strips or grids can be laid over the exposed cortex or introduced from the edges of the craniotomy over or under adjacent lateral, inferior, and medial cortical regions.
A common practice is to use holder-supported electrodes to record from the exposed cortex and Silastic-embedded electrodes to explore more remote cortical areas simultaneously.109 During temporal lobe surgery, single or multicontact needle electrodes are often inserted freehand transcortically into the regions of the amygdala and hippocampus that are inaccessible
by noninvasive means.34,46,48,59,63,86,104 An alternative approach to hippocampal, but not amygdalar, recording requires making either an incision in the lateral temporal cortex or an anterolateral temporal lobe resection to expose the hippocampus through the opened lateral ventricle and allow placement of individual electrodes59,60,75 or a multicontact Silastic strip86,110 over its ventricular surface. Various reference leads can be used for intraoperative brain recordings, including two interconnected electrodes on both sides of the neck109,110 or an alligator clip attached to a muscle near the edge of the craniotomy. Grounding of the patient usually is provided by a metal clamp attached to the post of the electrode holder or to the bone edge of the craniotomy. The ECoG electrode sets require cleaning and appropriate sterilization after each surgery to prevent transmission of agents highly resistant to decontamination,73 whereas commercial Silastic-embedded and intracerebral electrodes generally are disposable.
by noninvasive means.34,46,48,59,63,86,104 An alternative approach to hippocampal, but not amygdalar, recording requires making either an incision in the lateral temporal cortex or an anterolateral temporal lobe resection to expose the hippocampus through the opened lateral ventricle and allow placement of individual electrodes59,60,75 or a multicontact Silastic strip86,110 over its ventricular surface. Various reference leads can be used for intraoperative brain recordings, including two interconnected electrodes on both sides of the neck109,110 or an alligator clip attached to a muscle near the edge of the craniotomy. Grounding of the patient usually is provided by a metal clamp attached to the post of the electrode holder or to the bone edge of the craniotomy. The ECoG electrode sets require cleaning and appropriate sterilization after each surgery to prevent transmission of agents highly resistant to decontamination,73 whereas commercial Silastic-embedded and intracerebral electrodes generally are disposable.
In most centers, the ECoG is recorded digitally with a 16- or 21-channel electroencephalograph ideally located in a gallery adjacent to, but separate from, the operating room.54 A large glass window enables the clinical neurophysiologist to view the operating field, and an intercom system provides verbal communication between the neurophysiologist and the neurosurgeon. Photographs of the operating field can be taken through a tilted mirror. A recording system bandpass of 0.5 to 70 Hz (-3 dB) ensures appreciation of both epileptiform discharges and background activities. Further limiting the low-frequency response of the instrument is neither necessary nor justified except during electrical stimulation. In the course of this procedure, a low-frequency cutoff of 1.6 Hz or even 5.0 Hz helps to diminish the duration of amplifier blocking following discontinuation of the stimulus. Because activities recorded directly from the cortical surface are about 2 to 60 times larger than those detected on the scalp,1 instrumental sensitivities of 10 to 50 μV/mm are most commonly used for ECoG recordings. Modifications of the traditional instrumentation and physical arrangement for ECoG are suggested later in this chapter.
Recording and Electrical Stimulation
Preresection ECoGs survey, in a systematic order, cortical regions within and outside the area of exposure. In patients undergoing temporal lobectomy, recordings must include exploration of infratemporal and orbital frontal cortices, the hippocampus, and the amygdala; ECoGs omitting the assessment of these structures are both inadequate and potentially misleading.109,110 Postresection ECoGs examine the cortices surrounding the ablation as well as more distant structures. Following temporal lobectomy, these typically include the remnants of the hippocampus and parahippocampal gyrus, the insula, and neighboring extratemporal cortices. Total duration of preresection and postresection ECoGs, including monitoring of the effects of electrical stimulation but excluding functional mapping, is generally about 1 to 2 hours and sometimes longer. Both before and after resection, ECoGs can be obtained either bipolarly or referentially, but recording referentially expedites the interpretation and improves its accuracy.
Protocols followed in most centers for intraoperative electrical stimulation designed to elicit afterdischarges (Fig. 2), reproduce clinical manifestations of the patient’s habitual attacks, or both entail delivery of 0.5- to 2-msec diphasic pulses applied at 50 to 60 Hz over 1 to 5 seconds between two ball electrodes that terminate a pencil-shaped probe held by the surgeon.8 The stimulus can also be delivered between adjacent Silastic-enclosed electrodes. Individual stimulus trains are separated by at least 15 seconds,3 but longer intervals up to several minutes are necessary following a prolonged afterdischarge producing temporary refractoriness.3 Different stimulus parameters can be used for intraoperative functional mapping (Chapter 174).
Activation and Effects of Anesthesia
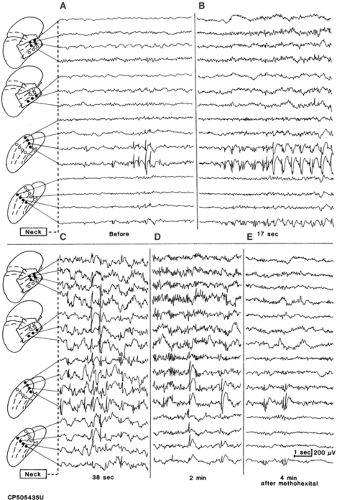
When no or rare interictal spikes are detected in the preresection ECoG, “activation” techniques can be used to elicit or enhance them. These procedures commonly consist of the intravenous administration of a general anesthetic such as thiopental sodium,18,34 methohexital,11,39 etomidate,43 or propofol.52 In the appropriate dose, these agents often cause the appearance or increase the rate of interictal spiking. However, the area displaying spikes, if any were present before the injection, frequently expands to wider cortical regions, and focal discharges can appear in noncontiguous, previously silent areas outside the anticipated margins of the resection. Thus, some results of the activation used with these agents, if not critically appraised, can encourage excessively wide surgical excisions. However, in a proportion of cases, the activation is restricted to a limited cortical area shortly after the injection, becomes again confined to this region as the action of the drug declines, or both (Fig. 3).10 In these instances, it is possible that the site displaying the earliest and the last drug-induced activation represents the patient’s epileptogenic area, although proof of the correctness of this inference is lacking. Over the last several years newer agents, specifically the short-acting opioids alfentanil71,91 and remifentanil,117 have been introduced for activation purposes during ECoG and may show greater promise for reliably identifying the epileptogenic zone, specifically in cases of temporal lobe epilepsy. Alfentenil has been reported to result in marked increases in spikes in hippocampus and parahippocampal regions and, to a lesser extent, in basal and lateral temporal cortex.91 Electrographic seizures triggered by this agent have also been shown to be concordant with spontaneously recorded clinical seizures.91 On the other hand, remifentanil can increase spikes in epileptogenic zone while suppressing discharges in the surrounding “normal” brain.117 Nevertheless, in the absence of unequivocal criteria reliably differentiating between drug-induced spiking indicative of an epileptogenic area to be removed and spurious drug effects that can be safely neglected, the results of activation procedures should be interpreted with caution.
General anesthetics can influence epileptiform activity not only when rapidly injected intravenously to activate the EcoG, but also when used in relatively stable concentrations to produce general anesthesia.66 However, even when anesthetics do suppress spikes, the intraoperative ECoG may still reliably reflect the awake interictal spiking pattern, provided that the spike frequency is >1 spike/min.10 The opioid analgesics commonly administered during epilepsy surgery, whether performed under local or general anesthesia, can influence interictal spiking and offer no clear advantages over fentanyl.20,45,66 On the other hand, neither propofol102 nor nitrous oxide51 is described as interfering with the awake ECoG. Large-dose propofol used as the sole anesthetic does not appear to trigger electrographic seizures in epilepsy patients.25 These effects should be taken into consideration in choosing the anesthetic and analgesic agents to be used during epilepsy surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree