Introduction and overview
The nervous system of all animals functions to detect changes in the internal and external environments and to bring about appropriate responses in muscles, organs and glands. With ascent of the evolutionary scale there is, in addition, an increasing capacity for ‘higher functions’ of the nervous system, such as learning, memory, cognition and, ultimately, self-awareness, intellect and personality. At the pinnacle of this process, the human nervous system is the most complex and versatile product of evolution.
While a great deal is known about how the nervous system works, much still awaits elucidation. Indeed, the anatomical, physiological, biochemical and molecular basis of neural function remain areas of intense research activity in both the basic and clinical sciences.
The nervous system can be damaged by inherited and developmental abnormalities, by disease and traumatic injury. The prevention, diagnosis and treatment of neurological disorders are, therefore, of immense socioeconomic importance. A knowledge of neuroanatomy and its correlation with function and dysfunction is fundamental to the practice of clinical neurosciences and to the prospect of future advances in the prevention and treatment of neurological disorders.
Neuroanatomical terminology and conventions
The formal names given to parts of the body are agreed internationally and have been published as the Terminologia Anatomica (1998; Thieme) by the Federative Committee on Anatomical Terminology (Fig. 1.1). The names of many structures in the nervous system have a Latin or Greek origin and are unique to neuroanatomy. Often the name is descriptive of some perceived physical characteristic, such as shape (e.g. ‘hippocampus’, meaning seahorse) or colour (e.g. ‘substantia nigra’, meaning black substance). Some structures are known by eponyms, usually in recognition of the person who first described them or whose work on them was particularly prominent (e.g. circle of Willis; foramen of Monro). Many of the latter names are considered to be anachronistic, but some remain in common usage.
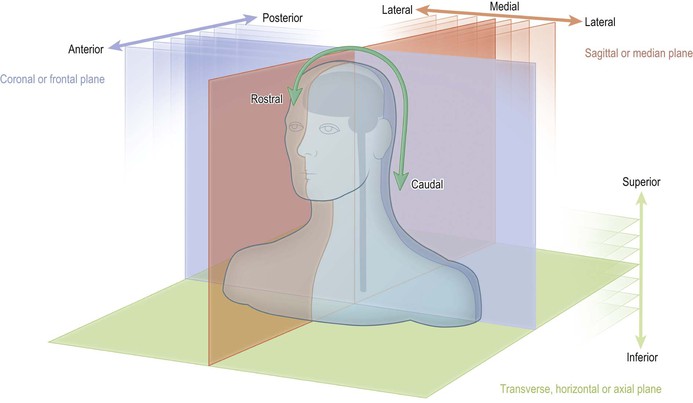
In anatomy generally, the location and relationships of structures are described with reference to three orthogonal planes: sagittal or median, horizontal or transverse (called axial in radiology) and coronal or frontal (Fig. 1.1). Directions and relationships are designated as medial or lateral, superior or inferior and anterior or posterior, with reference to the orientation of these planes. There is, however, an additional terminological complication when describing the brain and spinal cord, as explained below.
In neuroanatomy, the positional/directional terms: rostral, caudal, dorsal and ventral, are also commonly used. These terms have their origin in embryology and mean, respectively, towards the head end (rostral), tail end (caudal), back (dorsal) and belly (ventral). If the long axis of the brain and spinal cord were to remain in a straight line during embryological development then, in the adult, rostral would simply equate to superior, caudal to inferior, dorsal to posterior and ventral would equate to anterior. For all intents and purposes, this is what happens with the spinal cord but the long axis of the brain, on the other hand, undergoes considerable distortion and, in particular, the brainstem becomes flexed at several points (Fig. 1.12). In neuroanatomy, therefore, it is customary to use the terms that are common to all anatomy when describing a position in space and to reserve the terms ‘rostral, caudal, dorsal and ventral’ for the description of location and direction relative to the long axis of the nervous system.
In neuroanatomy, horizontal or transverse sections through the spinal cord and lower part of the brain (brainstem) are usually depicted/orientated with dorsal at the top and ventral at the bottom (Fig. 1.2A). The convention in neuroradiology, on the other hand, is that axial images are orientated as if looking from the subject’s feet towards the head, with anterior at the top of the image. In sections that contain the brainstem, therefore, the dorsal aspect of the brainstem is towards the bottom of the image and the ventral aspect is towards the top. This convention means that left and right are also reversed (Fig. 1.2B).
Components and organisation of the nervous system
Neurones and neuroglia
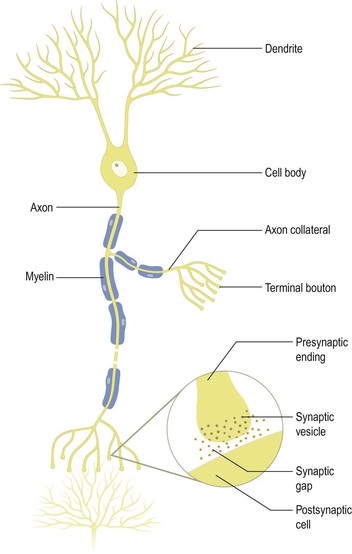
The basic structural and functional unit of the nervous system is the nerve cell or neurone (Figs 1.3, 1.4), of which the human nervous system is estimated to contain about 1010. The functions of the neurone are to receive and integrate incoming information from sensory receptors and other neurones and to transmit information to other neurones or non-neural structures that are under neural control (effector organs). Neuronal structure is highly specialised to fulfil these functions. Each neurone is a separate physical entity with a limiting cell membrane. Information is passed between neurones at specialised regions called synapses where the membranes of adjacent cells are in close apposition (Fig. 1.3).
There is wide diversity in the size and shape of neurones in different parts of the nervous system, but all share certain common characteristics. There is a single cell body from which a variable number of branching processes emerge. Most of these processes are receptive in function and are known as dendrites. The dendrites possess synapses, sometimes many thousands of them, through which they receive incoming information from other nerve cells. In sensory neurones, the dendrites may be further specialised to detect changes in the external or internal environment. One of the processes attached to the cell body is called the axon (nerve fibre) and this carries information away from the cell body. Axons are highly variable in length and may divide into several branches, or collaterals, through which information can be distributed to a number of different destinations simultaneously. At the end of the axon, synaptic specialisations called nerve terminals (presynaptic endings; terminal boutons) occur, from which information is transferred usually to the dendrites of other neurones. In efferent or motor neurones, which control non-neural structures such as muscle cells, the axonal endings may be further specialised (e.g. the neuromuscular junction).
Information is coded and distributed within neurones by changes in electrical charge. The cell membrane of neurones is polarised, which means that an electrical potential difference (the membrane potential) exists across it. In the resting state, this potential difference (the resting potential) is of the order of 60–70 millivolts (mV), the inside of the cell being negative with respect to the outside. When a neurone is stimulated or excited above a certain threshold level, there is a brief reversal of the polarity of its membrane potential, termed the action potential. Action potentials are propagated down the axon and invade the nerve terminals. At most synapses, transmission of information between neurones occurs by chemical rather than electrical means. Invasion of nerve terminals by an action potential causes the release of specific chemicals (neurotransmitters) that are stored in synaptic vesicles in the presynaptic ending. The neurotransmitter diffuses across the narrow gap between pre- and postsynaptic membranes and binds to specific receptors on the postsynaptic cell, inducing changes in the membrane potential. The change may be either to depolarise the membrane, thus moving towards the threshold for production of action potentials, or to hyperpolarise and, thus, stabilise the cell.
The other major cellular components of the nervous system are neuroglial cells, or glia, which outnumber neurones by about an order of magnitude. Unlike neurones, neuroglia do not have a direct role in information processing but they fulfil a number of other roles that are essential for the normal functioning of the nervous system.
Central and peripheral nervous systems
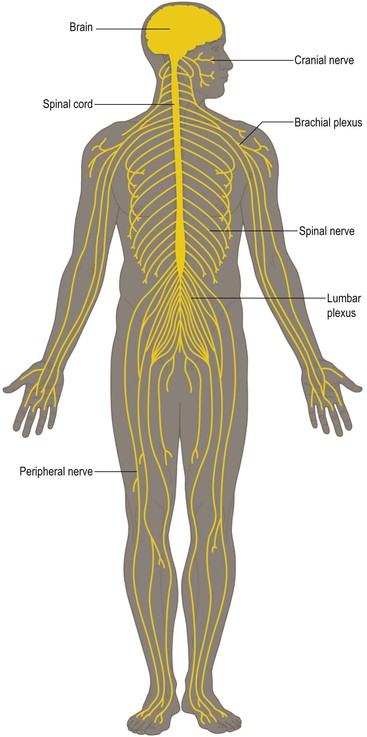
At a simple anatomical level, the nervous system (Fig. 1.5) is divided into the central nervous system (CNS) and the peripheral nervous system (PNS). The central nervous system consists of the brain and spinal cord, lying within the protection of the cranium and vertebral column, respectively. It is the most complex part of the nervous system, containing the majority of nerve cell bodies and synaptic connections. The peripheral nervous system constitutes the link between the CNS and structures in the periphery of the body, from which it receives sensory information and to which it sends controlling impulses. The peripheral nervous system consists of nerves joined to the brain and spinal cord (cranial and spinal nerves) and their ramifications within the body. Spinal nerves serving the upper or lower limbs coalesce to form the brachial or lumbar plexus, respectively, within which fibres are redistributed into named peripheral nerves. The PNS also includes many peripherally located nerve cell bodies, some of which are aggregated within structures called ganglia.
Somatic and autonomic nervous systems
At a functional level, neurones that are concerned with detecting changes in the external environment, or with the control of movement, are collectively referred to as the somatic nervous system. Neurones that detect changes in, and control the activity of, the viscera are collectively referred to as the autonomic nervous system. Somatic and autonomic components are present in both the central and peripheral nervous systems. The autonomic nervous system is divided into two anatomically and functionally distinct parts, namely the sympathetic and parasympathetic divisions, which generally have opposing (antagonistic) effects on the structures that they innervate. The autonomic nervous system innervates smooth muscle, cardiac muscle and secretory glands. It is an important part of the homeostatic mechanisms that control the internal environment of the body.
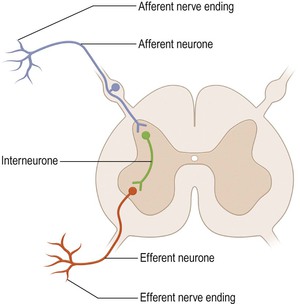
Afferent neurones, efferent neurones and interneurones
Nerve cells that carry information from peripheral receptors to the CNS are referred to as afferent neurones (Fig. 1.6). If the information they carry reaches consciousness, they are also called sensory neurones. Efferent neurones carry impulses away from the CNS and if they innervate skeletal muscle to cause movement, they are also called motor neurones. The vast majority of neurones, however, are located entirely within the CNS and are referred to as interneurones. The terms ‘afferent’ and ‘efferent’ are also commonly used to denote the polarity of projections to and from structures within the CNS, even though the projections are entirely contained within the brain and spinal cord. The projections to and from the cerebral cortex, for example, are referred to as cortical afferents and efferents, respectively.
Grey and white matter, nuclei and tracts
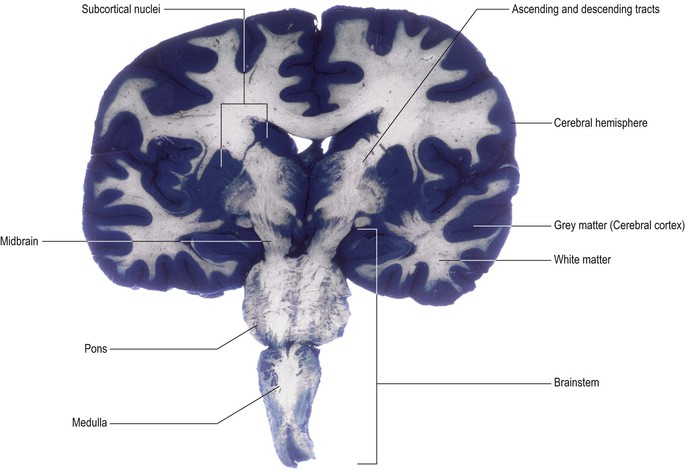
The CNS is a highly heterogeneous structure in terms of the distribution of nerve cell bodies and their processes (Fig. 1.7). Some regions are relatively enriched in nerve cell bodies (e.g. the central portion of the spinal cord and the surface of the cerebral hemisphere) and are referred to as grey matter. Conversely, other regions contain mostly nerve processes (usually axons). These are often myelinated (ensheathed in myelin), which confers a paler coloration – hence the term white matter.
Nerve cell bodies with similar anatomical connections and functions (e.g. the motor neurones innervating a group of related muscles) tend to be located together in groups called nuclei. Similarly, nerve processes sharing common connections and functions tend to follow the same course, running in pathways or tracts (Fig. 1.7 and see Fig. 1.23).
Decussation of sensory and motor pathways
It is a general principle of the organisation of the CNS that pathways conveying sensory information to a conscious level (the cerebral hemisphere) cross over, or decussate, from one side of the CNS to the other. The same is true of descending pathways from the cerebral hemisphere that control movement. Therefore, in general, each cerebral hemisphere perceives sensations from, and controls the movements of, the opposite (contralateral) side of the body.
Development of the central nervous system
By the beginning of the second week of human embryonic development, three germ cell layers become established: ectoderm, mesoderm and endoderm. Subsequently, these each give rise to particular tissues and organs in the adult. The ectoderm gives rise to the skin and the nervous system. The mesoderm forms skeletal, muscular and connective tissues. The endoderm gives rise to the alimentary, respiratory and genitourinary tracts.
The process of formation of the embryonic nervous system is referred to as neurulation. During the third week of embryonic development, the dorsal midline ectoderm undergoes thickening to form the neural plate (Figs 1.8, 1.9). The lateral margins of the neural plate become elevated, forming neural folds on either side of a longitudinal, midline depression, called the neural groove. The neural folds then become apposed and fuse together, thus sealing the neural groove and creating the neural tube. Some cells from the apices of the neural folds become separated to form groups lying dorsolateral to the neural tube. These are known as the neural crests. The formation of the neural tube is complete by about the middle of the fourth week of embryonic development.
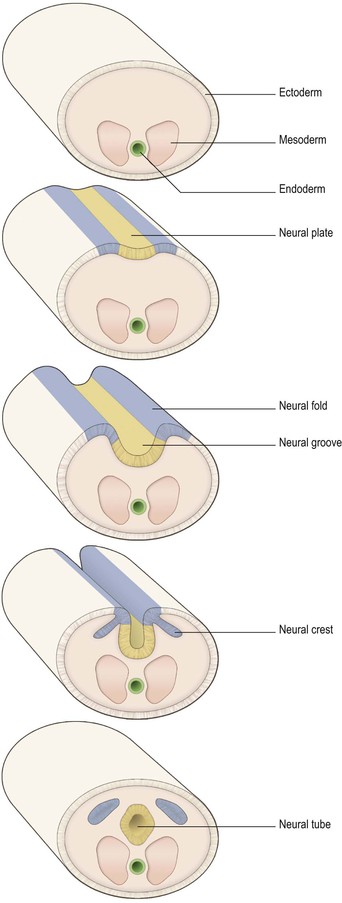
Enormous growth, distortion and cellular differentiation occur during the subsequent transformation of the neural tube into the adult CNS. This is maximal in the rostral part, which develops into the brain, the caudal portion becoming the spinal cord. The central cavity within the neural tube becomes the central canal of the spinal cord and the ventricles of the brain. The neural crests form the sensory ganglia of spinal and cranial nerves, and also the autonomic ganglia.
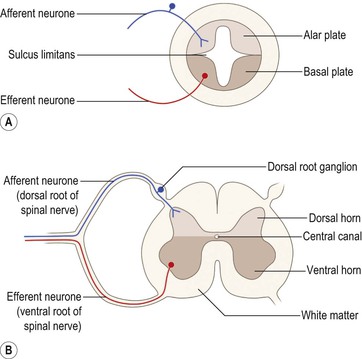
As development continues, a longitudinal groove, the sulcus limitans, appears on the inner surface of the lateral walls of the embryonic spinal cord and caudal part of the brain (Fig. 1.10A). The dorsal and ventral cell groupings thus delineated are referred to as the alar plate and the basal plate, respectively. Nerve cells that develop within the alar plate have predominantly sensory functions, while those in the basal plate are predominantly motor.
Further development also brings about the differentiation of grey and white matter. The grey matter is located centrally around the central canal, with white matter forming an outer coat. This basic developmental pattern can still easily be recognised in the adult spinal cord (Fig. 1.10B).
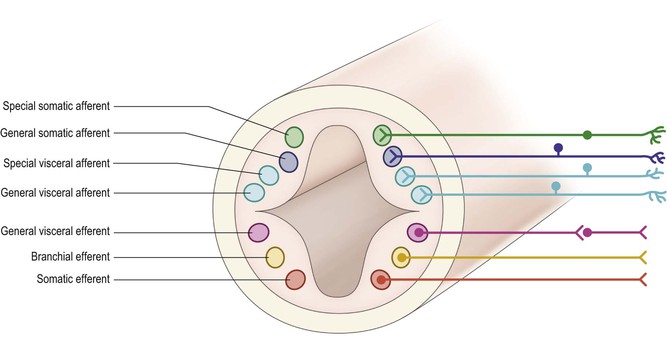
Further differentiation distinguishes seven neuronal cell groupings within the alar and basal plates (Fig. 1.11). These are arranged in discontinuous longitudinal columns, based upon their anatomical connections and physiological roles:
▪ General somatic afferent: receiving general sensory input from the periphery
▪ Special visceral afferent: subserving the sense of taste
▪ General visceral afferent: receiving afferent input from the viscera
▪ General visceral efferent: comprised of preganglionic autonomic efferents
▪ Branchial efferent: containing motor neurones to muscles derived from branchial (pharyngeal) arches
▪ Somatic efferent: containing motor neurones to somatic muscles.
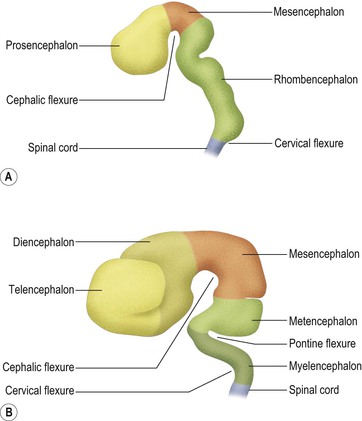
During embryonic development, the rostral portion of the neural tube undergoes massive differentiation and growth to form the brain (Fig. 1.12). By about the fifth week, three so-called primary brain vesicles can be identified: the prosencephalon (forebrain), mesencephalon (midbrain) and rhombencephalon (hindbrain). The longitudinal axis of the developing CNS (neuraxis) does not remain straight but is bent by a midbrain or cephalic flexure, occurring at the junction of midbrain and forebrain, and a cervical flexure between the brain and the spinal cord.
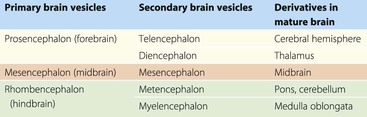
By the seventh week, further differentiation distinguishes five secondary brain vesicles produced by division of the prosencephalon into the telencephalon and diencephalon and division of the rhombencephalon into the metencephalon and myelencephalon. The junction between the latter is marked by an additional bend in the neuraxis, called the pontine flexure.
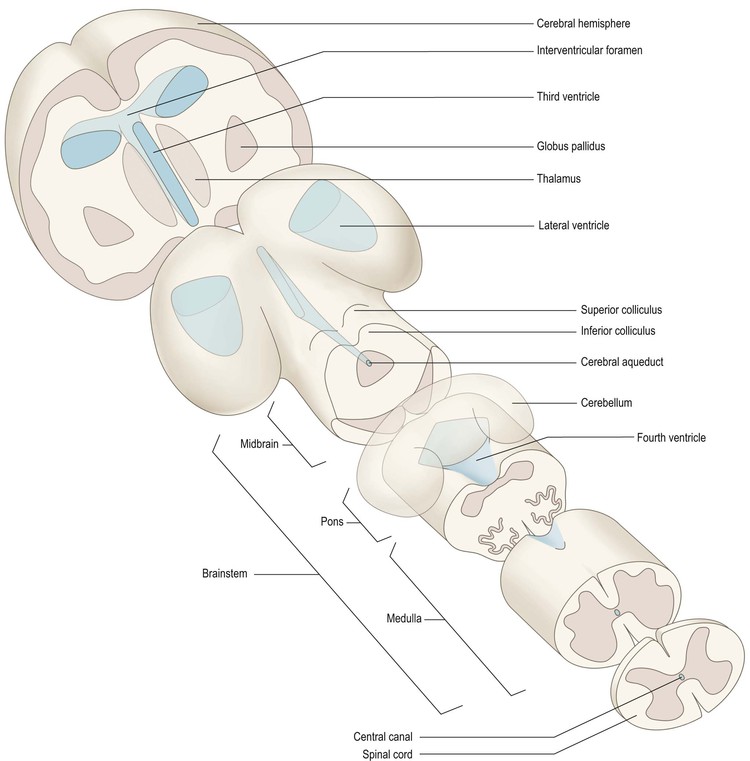
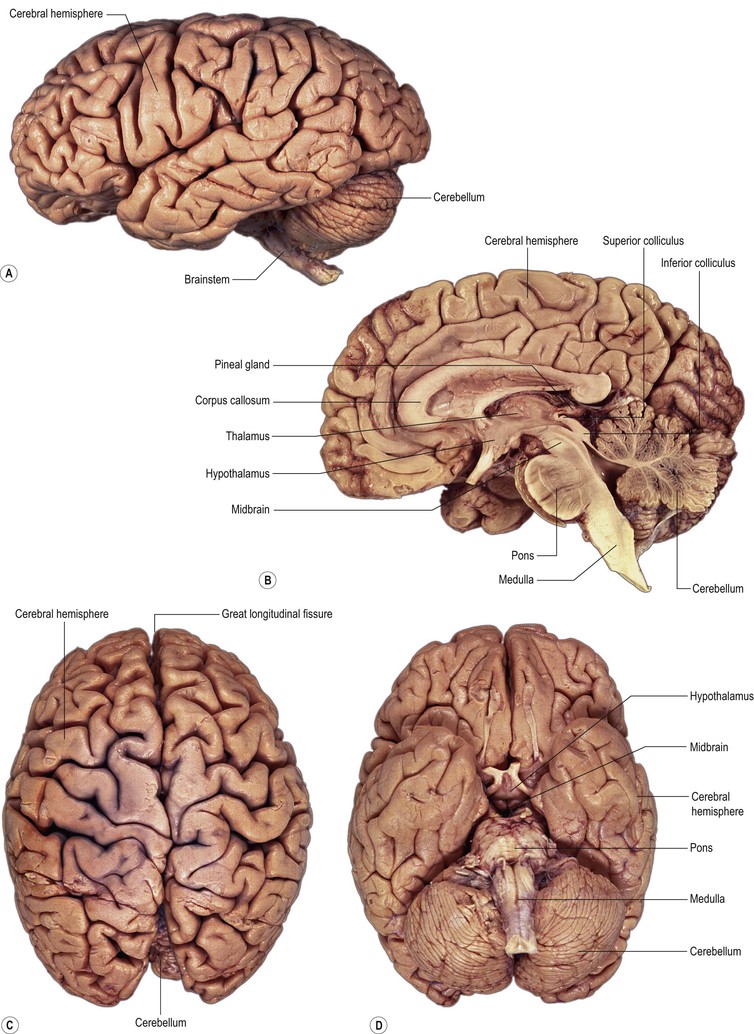
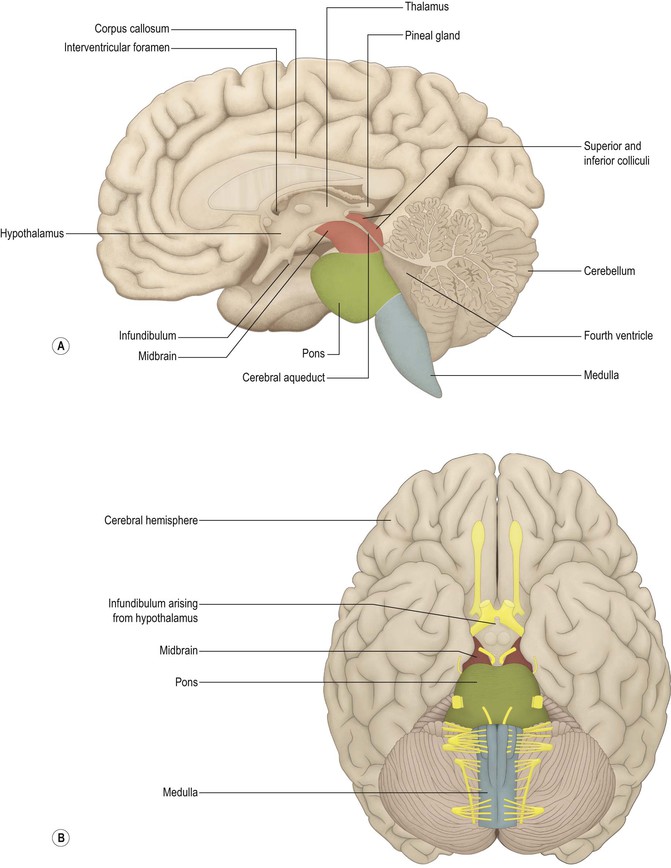
Some of the names of the embryological subdivisions of the brain are commonly used for descriptive purposes and it is, therefore, useful to know the parts of the mature brain into which they subsequently develop (Table 1.1). Of the three basic divisions of the brain, the prosencephalon or forebrain is by far the largest. It is also referred to as the cerebrum. Within the cerebrum, the telencephalon undergoes the greatest further development and gives rise to the two cerebral hemispheres. These consist of an outer layer of grey matter (the cerebral cortex) and an inner mass of white matter, within which various groups of nuclei lie buried (the largest being the corpus striatum). The diencephalon consists largely of the thalamus, which contains numerous cell groupings and is intimately connected with the cerebral cortex. The mesencephalon, or midbrain, is relatively undifferentiated (it still retains a central tube-like cavity surrounded by grey matter). The metencephalon develops into the pons and overlying cerebellum, while the myelencephalon forms the medulla oblongata (medulla). The medulla, pons and midbrain are collectively referred to as the brainstem (Fig. 1.13).
As the brain develops, its central cavity also undergoes considerable changes in size and shape, forming a system of chambers or ventricles (Fig. 1.13 and see Fig. 1.22), which contain cerebrospinal fluid.
Parallels have been drawn between the embryological development of the brain and the major changes that the brain has undergone during ascent of the phylogenetic, or evolutionary, scale from simple to more complex animals. While this is certainly an oversimplification, the concept does have the educational merit of introducing some of the principal parts of the brain, and their relationships to one another, in a graphic and memorable way (Fig. 1.13).
The simplest of chordate animals (e.g. amphioxus), from which the vertebrates evolved, possess a dorsal tubular nerve cord that is reminiscent of the neural tube of the developing mammalian embryo. During phylogeny, the rostral end of the tubular nervous system has undergone enormous modification and change; consequently, the adult human brain bears little obvious similarity to its evolutionary ancestors.
Regional specialisation has been an important theme in the evolution of the brain and this is especially obvious in relation to the senses and in movement control. Long ago in phylogeny, centres devoted to these functions developed as expansions or outgrowths from the dorsal aspect of the simple tubular brain (Fig. 1.13). In form, they consisted of an outer cortex of nerve cell bodies with an underlying core of nerve fibres. Bilaterally paired centres developed in relation to the senses of smell, vision and hearing, and a symmetrical, midline centre developed in association with vestibular function and the maintenance of equilibrium. Each of these centres underwent subsequent evolutionary change, but this was most evident in the rostral, ‘olfactory’, part of the brain, which developed into the massive cerebral hemispheres (Figs 1.14, 1.15). During this process, known as prosencephalisation, the cerebral hemispheres came to take on an executive role in many areas of brain function. For example, the highest level for the perception and interpretation of input from all sensory modalities eventually became localised in the cortical surface of the cerebral hemispheres, as did the highest level for motor control. This is reflected by the fact that only a small proportion of the adult human cerebral hemisphere remains devoted to olfactory function.
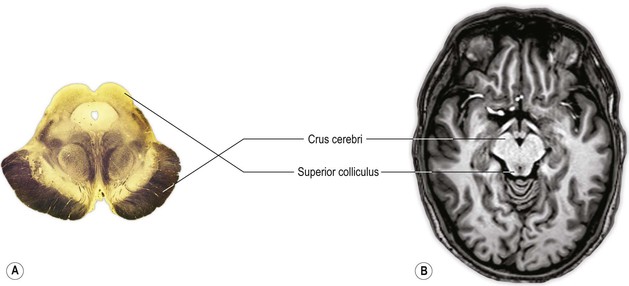
The process of prosencephalisation meant that the other integrative centres became progressively subservient to the cerebral hemispheres. For example, those for vision and hearing underwent relatively little further development and fulfil largely automatic, reflex functions in the human brain. They may still be identified, however, as four small swellings on the dorsal surface of the midbrain: the corpora quadrigemina or superior and inferior colliculi (Figs 1.13–1.15). The motor centre near the caudal end of the brain developed into the cerebellum (Figs 1.13–1.15), which retains a central role in the maintenance of equilibrium and the coordination of movement.
Overview of the anatomy of the central nervous system
Coverings and blood supply
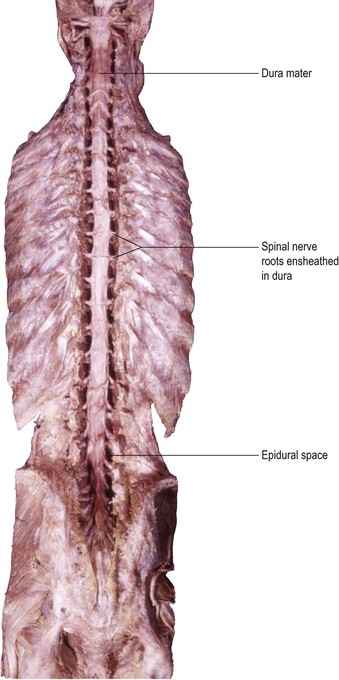
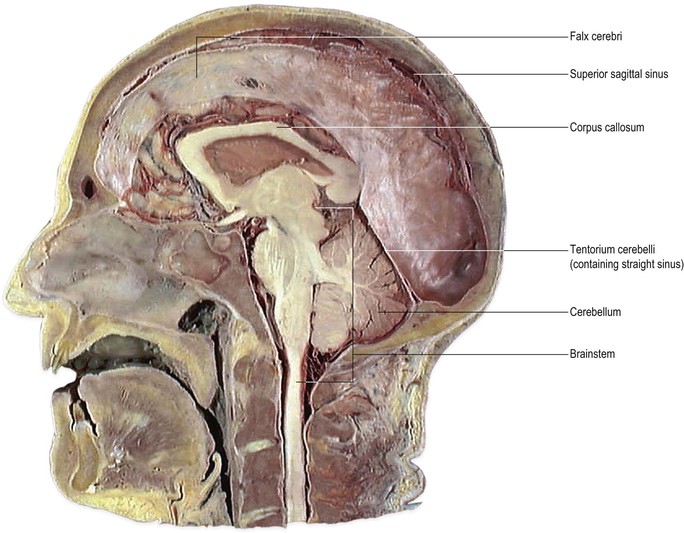
The brain and spinal cord are supported and protected by the bones of the skull and vertebral column, respectively. Within these bony coverings, the CNS is entirely ensheathed by three layers of membranes, called the meninges (Fig. 1.16). The outermost membrane is the dura mater, a tough, fibrous coat that surrounds the brain and spinal cord like a loose-fitting bag (Fig. 1.17). The spinal dura and much of the cranial dura are separate from the periosteum, which lines the surrounding bones. At certain locations, however, such as the floor of the cranial cavity, the dura and periosteum are fused and the cranial dura is tightly adherent to the interior of the skull. Two large sheets of dura project into the cranial cavity, incompletely dividing it into compartments (Fig. 1.18). The falx cerebri lies in the sagittal plane between the two cerebral hemispheres. Its free border lies above the corpus callosum. The tentorium cerebelli is oriented horizontally, lying beneath the occipital lobes of the cerebral hemispheres and above the cerebellum. The dura mater can be regarded as consisting of two layers. These are fused together except in certain locations, where they become separated to form spaces, the dural venous sinuses, which serve as channels for the venous drainage of the brain. Important dural sinuses occur:
▪ on the floor of the cranial cavity
▪ along the lines of attachment of the falx cerebri and tentorium cerebelli to the interior of the skull (superior sagittal sinus, Fig. 1.18; transverse sinus, Figs 7.9, 7.10)
▪ along the line of attachment of the falx cerebri and tentorium cerebelli to one another (straight sinus, Figs 7.9, 7.10).
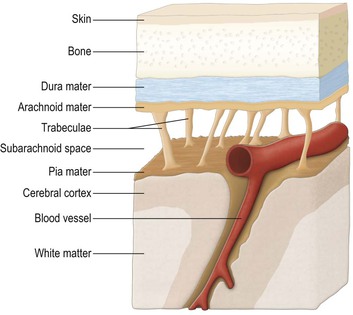
Beneath the dura lies the arachnoid mater, the two being separated by a thin subdural space. The arachnoid is a translucent, collagenous membrane that, like the dura, loosely envelops the brain and spinal cord. The innermost of the meninges is the pia mater, a delicate membrane of microscopic thickness that is firmly adherent to the surface of the brain and spinal cord, closely following their surface contours. Between the arachnoid and pia is the subarachnoid space through which CSF circulates.
The brain is supplied with arterial blood by the internal carotid and vertebral arteries, which anastomose to form the circulus arteriosus (circle of Willis) on the base of the brain. The spinal cord is supplied by vessels arising from the vertebral arteries, reinforced by radicular arteries derived from segmental vessels. The arteries and veins serving the CNS run for part of their course within the subarachnoid space (Fig. 1.16). The meninges are supplied by a number of vessels, the most significant intracranial one being the middle meningeal artery, which ramifies extensively between the skull and dura mater overlying the lateral aspect of the cerebral hemisphere.
Anatomy of the spinal cord
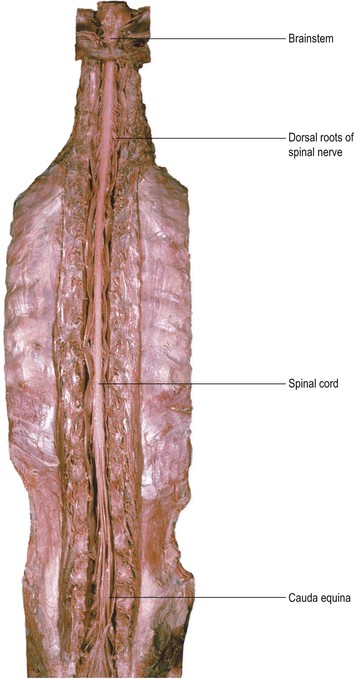
The spinal cord lies within the vertebral (spinal) canal of the vertebral column and is continuous rostrally (superiorly) with the medulla oblongata of the brainstem (Fig. 1.19). The spinal cord receives information from, and controls, the trunk and limbs. This is achieved through 31 pairs of spinal nerves that are attached to the cord at intervals along its length and contain afferent and efferent nerve fibres connecting with structures in the periphery. Near to the cord, the spinal nerves divide into dorsal (posterior) and ventral (anterior) roots, which attach to the cord along its dorsolateral and ventrolateral borders, respectively (Fig. 1.20). The dorsal roots carry afferent fibres, the cell bodies of which are located in dorsal root ganglia. The ventral roots carry efferent fibres with cell bodies lying within the spinal grey matter. Spinal nerves leave the vertebral canal through small holes, called intervertebral foramina, located between adjacent vertebrae. Because of a difference in the rates of growth of the spinal cord and vertebral column during development, the spinal cord in the adult does not extend the full length of the vertebral canal, but ends at the level of the intervertebral disc between L1 and L2. The lumbar and sacral spinal nerves, therefore, descend in a leash-like arrangement, the cauda equina (Fig. 1.19), to reach their exit foramina.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree