Ischemic Cerebrovascular Disease
José Biller
Cerebrovascular disease comprises a heterogeneous group of disorders that herald their presence by producing symptoms and signs resulting from either ischemia or hemorrhage within the CNS. The term stroke is most commonly used by both physicians and the general public to refer to any one of this diverse group of disorders. It connotes the notion that onset of symptoms is abrupt and leaves a lasting physical or cognitive disability. An ischemic stroke is an acute brain attack caused by the interruption of blood flow within one or more arterial territories of the brain.
Stroke is currently the second most common cause of death worldwide, and a primary cause of long-term disability in much of the industrialized world. Cerebrovascular disease is also the most common neurologic condition necessitating hospitalization. Patients surviving a transient ischemic attack (TIA) or ischemic stroke are at increased risks of recurrent ischemic events and have a reduced life expectancy.
There are two main types of strokes—ischemic and hemorrhagic. The focus of this chapter is to outline the general approach to the diagnosis and management of ischemic stroke.
Of the 795,000 new or recurrent strokes in the United States each year, approximately 87% result from cerebral infarction. On average, someone in the United States has a stroke every 40 seconds, and someone dies of a stroke every 4 minutes. Ischemic stroke may result from (1) large-artery atherosclerotic disease, (2) small-artery disease (lacunes), (3) cardioembolism, (4) hemodynamic (watershed) infarction, (5) nonatherosclerotic vasculopathies, (6) hypercoagulable disorders, or (7) infarction of undetermined causation.
Ischemia sets in motion a cascade of biochemical alterations leading to lactic acidosis, influx of calcium and sodium, and efflux of potassium that culminates in cell death. The pathogenesis of ischemic strokes can be conceptualized as a permanent lack of blood flow to a focal region of the brain, depriving it of needed glucose and oxygen. Normal cerebral blood flow (CBF) to the adult brain is 50 to 55 ml per 100 g per minute. The threshold for synaptic transmission failure occurs when CBF decreases to approximately 8 to 10 ml per 100 g per minute. At this level, neuronal death can occur. The brain region with a CBF level from 8 to 18 mL per 100 g per minute sometimes is called the ischemic penumbra. Rational treatment of patients with ischemic cerebrovascular disease depends on accurate diagnosis. Most interventional strategies aim to promote rapid perfusion of brain tissue and to treat the complications of brain swelling postischemic stroke.
The cause of an ischemic stroke must first be established through an expeditious but careful history-taking, detailed physical examination, and paraclinical investigations. A basic evaluation, to be performed for all patients with ischemic stroke, includes complete blood cell count with differential and platelet count, erythrocyte sedimentation rate, prothrombin time (PT), activated partial thromboplastin time (aPTT), plasma glucose level, blood urea nitrogen, serum creatinine, lipid analysis, urinalysis, chest radiography, and electrocardiography. Creatine kinase (CK) and CK MB fraction and cardiac troponins provide evidence of a concurrent myocardial infarction (MI).
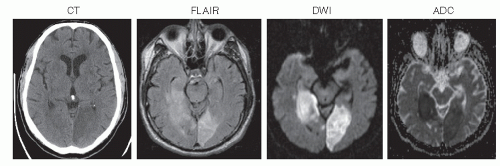
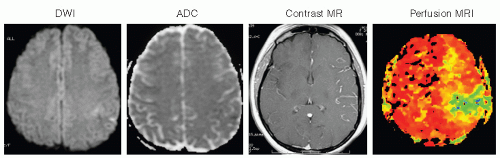
CT should also be performed on all patients because it may depict hemorrhagic lesions that can mimic an ischemic stroke. If cerebellar or brainstem symptoms are present, imaging should include thin cuts through the posterior fossa. MRI is superior to CT in evaluation for cerebral ischemia (Fig. 36.1). MRI with diffusion-weighted imaging (DWI) is useful to delineate ischemic strokes. MRI with DWI or perfusion-weighted imaging is useful to visualize the ischemic penumbra (Fig. 36.2). Computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) complements the information obtained
with MRI and frequently delineates the pathoanatomic substrate of the stroke. Gadolinium enhanced multicontrast-weighted MRI may also allow accurate quantification of carotid artery plaque instability. CT perfusion is also useful to visualize the ischemic penumbra. The emphasis in screening should be on noninvasive testing including carotid duplex ultrasound and transcranial Doppler ultrasound. Carotid duplex ultrasound is often obtained when clinical manifestations could be attributed to carotid artery disease. Increased plaque echolucency has been associated with higher risks of stroke and TIA. Transcranial Doppler ultrasound identifies high-risk patients with sickle cell anemia, indirectly assesses cerebral perfusion reserve, and may also enhance the effect of ultrasound on thrombolysis.
with MRI and frequently delineates the pathoanatomic substrate of the stroke. Gadolinium enhanced multicontrast-weighted MRI may also allow accurate quantification of carotid artery plaque instability. CT perfusion is also useful to visualize the ischemic penumbra. The emphasis in screening should be on noninvasive testing including carotid duplex ultrasound and transcranial Doppler ultrasound. Carotid duplex ultrasound is often obtained when clinical manifestations could be attributed to carotid artery disease. Increased plaque echolucency has been associated with higher risks of stroke and TIA. Transcranial Doppler ultrasound identifies high-risk patients with sickle cell anemia, indirectly assesses cerebral perfusion reserve, and may also enhance the effect of ultrasound on thrombolysis.
Cardiac investigations to determine whether emboli have cardiac sources are advised in selected circumstances. Two-dimensional echocardiography for older patients with ischemic stroke is limited to patients with clinical clues of heart disease. Two-dimensional echocardiography should be considered for all patients younger than 45 years with otherwise unexplained ischemic stroke. Transesophageal echocardiography should be used for selected individuals, particularly for evaluation of mitral and aortic prosthetic valves or vegetations, whenever there is a need for better visualization of the left atrial appendage or interatrial septum, or when a right-to-left shunt is suspected.
Most patients with ischemic stroke have extracranial or intracranial cerebrovascular atherosclerosis. Cerebrovascular atherosclerosis primarily affects the carotid bulb, carotid siphon, middle cerebral artery (MCA) stem, origin, and intracranial segments of the vertebral arteries, and basilar artery. Ischemia results from thrombotic vascular occlusion, embolization of atherosclerotic debris, or hemodynamic disturbances causing focal hypoperfusion in areas in which the circulation is inadequate.
I. NATURAL HISTORY AND PROGNOSIS
A. Ischemic stroke resulting from large-artery atherosclerotic disease.
Large-artery atherothrombotic infarctions almost always occur in patients who already have sig nificant risk factors for cerebrovascular atherosclerosis, such as arterial hypertension, cigarette smoking, diabetes mellitus, dyslipidemia, asymptomatic carotid bruits, asymp tomatic carotid stenosis, and TIAs.
1. A TIA is defined as a transient episode of neurologic dysfunction caused by focal brain or retinal ischemia without infarction. The conventional boundary in differentiating between a TIA and stroke has been 24 hours. Yet, most TIAs last only a few min utes. TIAs are most often caused by thromboembolism associated with large artery atherosclerosis, cardioembolism, or small vessel (lacunar) disease. TIAs are important harbingers of subsequent stroke and are often associated with lesions on brain imaging. TIAs involving the anterior or carotid circulation should be distinguished from those involving the posterior or vertebrobasilar circulation.
a. The following symptoms are considered typical of TIAs in the carotid circulation: ipsilateral amaurosis fugax, contralateral sensory or motor dysfunction limited to one side of the body, aphasia, contralateral homonymous hemianopia, or any combina tion thereof.
b. The following symptoms represent typical TIAs in the vertebrobasilar system: bilateral or shifting motor or sensory dysfunction, complete or partial loss of vision in both homonymous fields, or any combination of these symptoms. Isolated diplo pia, vertigo, dysarthria, and dysphagia should not be considered TIAs, but in com bination with one another or with any of the symptoms just listed, they should be considered vertebrobasilar TIAs.
c. Preceding TIAs occur in approximately 30% to 50% of patients with atherothrom botic brain infarction, in 15% to 25% of lacunar infarctions, and in 10% of cardioembolic infarctions.
d. A TIA is a risk factor for stroke. The independent risk of a subsequent stroke is at least three times greater for patients with histories of TIAs than for those who have not had TIAs. The ABCD2 score is useful for stroke risk stratification in patients with TIAs: Age > 60 years = 1 point; Blood pressure > 140/90 mm Hg = 1 point; Clinical unilateral weakness = 2 points; speech-only impairment = 1 point; Duration > 60 min utes = 2 points, < 60 minutes = 1 point; Diabetes = 1 point. Scores of 4 or greater in the ABCD2 score indicate moderate to high-stroke risk.
2. Atherosclerosis tends to occur in areas of reduced flow shear such as the posterior aspect of the carotid artery bulb. It primarily affects the larger extracranial and intracranial vessels. Approximately 80% of ischemic strokes occur in the carotid or anterior circulation, and 20% in the vertebrobasilar or posterior circulation (Fig. 36.3).
3. The mechanism of large-artery atherothrombotic infarction is either artery-to-artery embolization or in situ formation of a thrombus in the setting of preexisting arterial stenosis. Artery-to-artery embolism or low-CBF is a common mechanism of cerebral ischemic events. Embolism from ulcerated carotid atherosclerotic plaques is the most common cause of cerebral infarction. In situ thrombosis occurs in the proximal carotid, distal vertebral, and basilar arteries. Such a circumstance may arise in association with hypercoagulable states. When the internal carotid artery (ICA) occludes, it can also cause low-flow ischemic events depending on status of the collateral circulation.
B. Ischemic strokes resulting from small-vessel or penetrating artery disease (lacunes).
Long-standing arterial hypertension affects primarily the smaller penetrating intracranial vessels. It induces hypertrophy of the media and deposition of fibrinoid material into the vessel wall (fibrinoid necrosis), which eventually leads to occlusion. Lacunes are small ischemic infarcts in the deep regions of the brain or brainstem rang ing in diameter from 0.5 to 15.0 mm resulting mainly from lipohyalinosis of penetrating arteries or branches related to long-standing arterial hypertension—chiefly the anterior choroidal, middle cerebral, posterior cerebral, and basilar arteries. Diabetes mellitus and extracranial arterial and cardiac sources of embolism are found less frequently.
TABLE 36.1 Cardiac Sources with High-Risk Embolic Potential | |||||||
|---|---|---|---|---|---|---|---|
|
C. Ischemic stroke resulting from cardioembolism.
Cardioembolic strokes are associated with substantial morbidity and mortality. Embolism of cardiac origin accounts for approximately 15% to 20% of all ischemic strokes. Emboli from cardiac sources frequently lodge in the MCA territory, often are large, and often have the worst outcomes. Although most types of heart disease can produce cerebral embolism, certain cardiac disorders are more likely to be associated with emboli (Table 36.1). Low or uncertain embolic risk disorders include mitral valve prolapse, mitral annulus calcification, aortic valve calcification, cal cific aortic stenosis, bicuspid aortic valve, atrial flutter, patent foramen ovale, atrial septal aneurysms, valvular strands, and a Chiari network. Identification of a cardiac source of potential embolism is helpful for management. However, finding a potential cardiac embolic source is not by itself sufficient to diagnose embolic cerebral infarction because many cardiac problems can coexist with cerebrovascular atherosclerosis.
D. Ischemic stroke resulting from hemodynamic mechanisms.
Another mechanism of ischemic CNS damage is decreased systemic perfusion pressure that causes diminished
blood flow to the brain in a diffuse manner. This occurs most commonly in the setting of cardiac pump failure or systemic hypotension. Border-zone ischemia often is explained by the combination of two frequently interrelated processes—hypoperfusion and embolization. This type of insult is most critical in border-zone territories, or socalled watershed areas, in the most distal regions of supply of the major arterial territories. Border-zone ischemia can result in several characteristic syndromes depending on whether the ischemia is in the border-zone territory of all three major arterial systems (anterior, middle, and posterior cerebral arteries), the territory between the anterior and middle cerebral arteries, or the territory between the middle and posterior cerebral arteries. Watershed infarcts often are bilateral, but can be unilateral when preexisting ipsilateral vascular disease causes focal hypoperfusion in the most distal territory. Other mechanisms whereby watershed infarcts develop include microemboli and hematologic abnormalities.
blood flow to the brain in a diffuse manner. This occurs most commonly in the setting of cardiac pump failure or systemic hypotension. Border-zone ischemia often is explained by the combination of two frequently interrelated processes—hypoperfusion and embolization. This type of insult is most critical in border-zone territories, or socalled watershed areas, in the most distal regions of supply of the major arterial territories. Border-zone ischemia can result in several characteristic syndromes depending on whether the ischemia is in the border-zone territory of all three major arterial systems (anterior, middle, and posterior cerebral arteries), the territory between the anterior and middle cerebral arteries, or the territory between the middle and posterior cerebral arteries. Watershed infarcts often are bilateral, but can be unilateral when preexisting ipsilateral vascular disease causes focal hypoperfusion in the most distal territory. Other mechanisms whereby watershed infarcts develop include microemboli and hematologic abnormalities.
E. Ischemic stroke resulting from nonatherosclerotic vasculopathies.
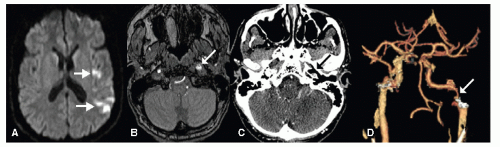
Several nonatherosclerotic forms of vasculopathy are predisposing factors for ischemic stroke. These vasculopathies include, among others, cervicocephalic arterial dissection (Fig. 36.4), Moyamoya, fibromuscular dysplasia, and cerebral vasculitis. Together, these uncom mon conditions represent 5% of all ischemic strokes. They are relatively more common among children and young adults.
F. Ischemic stroke resulting from hypercoagulable disorders.
Alterations in hemostasis have been associated with an increased risk of ischemic stroke. These conditions include deficiencies in the anticoagulant proteins such as antithrombin, protein C, protein S, activated protein C resistance, factor V Leiden mutation, prothrombin gene (G20210A) mutation, and heparin cofactor II; disorders of fibrinogen or of the fibri nolytic system; methylene-tetrahydrofolate reductase (MTHFR) gene mutation (par ticularly the MTHFR 677TT polymorphism); lipoprotein (a) disorders; and secondary hypercoagulable states encountered in patients with malignancies, pregnancy/puerperium, the antiphospholipid antibody syndrome, nephrotic syndrome, polycythemia vera, sickle cell disease, thrombotic thrombocytopenic purpura (TTP), and paroxysmal nocturnal hemoglobinuria. These disorders account for 1% of all strokes and for 2% to 7% of ischemic strokes in young patients.
G. Ischemic stroke of undetermined causation.
Despite extensive evaluation, in as many as one-third of ischemic strokes, a cause cannot be determined. This percentage is possibly higher among patients younger than 45 years. It is possible that some of these strokes are caused by cardioembolic or hematologic events not readily demonstrable.
II. PREVENTION
The prevention of strokes follows three main avenues—control of modifiable risk factors, pharmacologic therapy, and surgical intervention. Knowledge and control of modifiable risk factors are paramount in prevention of primary and secondary strokes. Treatable or modifiable risk factors include arterial hypertension, diabetes mellitus, cigarette smoking, hyperlipidemia, excessive alcohol intake, obesity, and physical inactivity. Other risk factors include age and gender, cardiac disease, TIAs, previous strokes, asymptomatic carotid bruit or stenosis, high hemoglobin level or hematocrit, increased fibrinogen level, use of oral contraceptives, and possibly race and ethnicity.
A. Hypertension
predisposes to ischemic stroke by aggravating atherosclerosis and accel erating heart disease. Approximately, 60 million Americans have arterial hypertension. Arterial hypertension is the most important modifiable risk factor for stroke, increasing the relative risk 3- to 4-fold. Blood pressure lowering also reduces the risk of stroke in individuals with isolated systolic hypertension and in elderly subjects. Blood pressure treatment resulting in a decrease in mean diastolic blood pressure of 5 mm Hg over 2 to 3 years is associated with a 40% reduction in risk of stroke. Blood pressure targets are individualized according to age, ethnicity, and coexisting comorbidities. Reduction of blood pressure is more important than the specific antihypertensive agent or modality use.
B. Diabetes mellitus
increases the risk of ischemic cerebrovascular disease 2- to 4-fold compared with the risk among persons without diabetes. In addition, diabetes increases morbidity and mortality after stroke. Most persons with diabetes die of atherosclerotic cardiovascular complications, and atherosclerosis causing blockage to the arteries in the heart and brain accounts for >80% of all deaths in diabetes. Rigorous blood pressure control is particularly recommended for diabetic patients. Patients with diabetes who have concomitant hypertension should be treated with a regimen that includes an ACE inhibitor or an angiotensin receptor blocker. There is presently no evidence to suggest that tighter diabetic control decreases the risk of stroke or recurrent stroke.
C. Cigarette smoking
is an independent risk factor for ischemic stroke among men and women of all ages. More than 5 years may be required before a reduction in stroke risk is observed after cessation of smoking.