34 Kyphoplasty
KEY POINTS
Brief Description
Spinal osteoporosis alone is asymptomatic. If allowed to progress, however, it confers increasing risk of fragility fracture. The principal manifestation of osteoporotic vertebral compression fractures (VCFs) is back pain. Some minimally symptomatic patients do not present for medical evaluation.1 Others require hospital admission for unrelenting pain. Typically, over 3 months, the fracture heals and the back pain subsides.2 Although the nonunion rate is low, not all VCFs heal.
Back pain can persist after fracture healing. From 33% to 75% of fractures precipitate chronic back pain.3 The chronic pain has been attributed to hyperkyphosis, leading to excessive muscular strain. Excessive anterior vertebral body loading engendered by this malalignment may propagate stress fractures in the surrounding endplates.4 Late kyphosis is occasionally associated with myelopathy.5
Indications and Contraindications
Over time, kyphoplasty indications have gradually been expanded to include conditions such as multiple myeloma and osteolytic metastases. Moreover, kyphoplasty has been added to open decompression and internal fixation procedures. Hybrid procedures may be indicated for more complex fracture patterns, significant compression of the neural elements, and neoplastic lesions with cortical destruction.7 Another hybrid option combines radiosurgery and kyphoplasty. Conventional radiotherapy remains the index treatment in many patients with vertebral body metastasis.7 Used alone, radiation is associated with delayed pain relief and further vertebral collapse due to both the previous bone erosion and the radiation itself. Newer radiation therapy techniques allow more focused radiation to be applied via intense treatments over a shorter time course.
Absolute contraindications to kyphoplasty include the following:
While less common than VCF, osteoporotic burst fractures (senile burst fractures) are not rare. Any fracture precipitating more than 50% height loss will have associated posterior cortical compromise. In many cases, this compromise takes the form of cortical buckling. When the canal occlusion is less than 33%, kyphoplasty can be considered. On the other hand, in the face of cortical comminution, avoid percutaneous kyphoplasty because of the increased risk of cement extravasation. In patients with neurologic injury, open surgery may be required.
Background of Scientific Testing and Clinical Outcomes
Outcomes data include a number of retrospective studies. Very recently prospective data have been reported from the FREE trial.10 This trial included 21 sites in 8 countries that enrolled 300 patients with acute VCF and randomized them to either kyphoplasty (149) or nonoperative care (151). As of this writing, the complete paper has not been published, but early pain relief seems to be a clear advantage of kyphoplasty. Whether that advantage persists is more difficult. The primary outcome was the difference in the Short Form (SF-36) physical component summary at 1 month. Quality of life measurements and spine radiographs were assessed through 12 months. Kyphoplasty subjects reported greater improvement than controls in their SF-36 physical component (5.2 point difference; p < 0001) at one month). By 12 months, the difference declined to 1.5 points and was no longer significant (p = .2). Kyphoplasty improved quality of life by the 1-point EuroQol questionnaire at 1 (0.18 points; 95% CI, 0.08–0.28; p < .001) and 12 (0.12; 95% CI, 0.01–0.22; p = .025) months. Back function, as measured by the 24-point Roland-Morris scale, was improved by 4.0 points by kyphoplasty at 1 month (p < .001) and 2.6 points at 12 months (p = .001). Kyphoplasty patients reported fewer days with limited activity, less back pain, and less use of analgesics and walking aids.
Of note, the FREE study was funded by the manufacturer and many of its authors are Kyphon consultants. On the other hand, three other small studies comparing kyphoplasty with conventional medical treatment also found that kyphoplasty consistently improved pain and physical function, with results sustained at 6 months.11–13
In 2005, Hadjipavlou et al14 combined the available vertebroplasty and kyphoplasty outcome reports in an effort to compare the procedures. Using meta-regression techniques, the authors found that individual study design had a considerable impact on subsequent analysis. For prospective studies, the rates of success with vertebroplasty and kyphoplasty were not significantly different at 92% and 93% respectively. However, in retrospective studies, kyphoplasty was more successful (95% vs. 86%; p = .019)
Aside from pain relief, a major benefit of VBA lies in the restoration of mobility. In one series of 11 wheelchair-bound cancer patients, 73% were able to walk shortly after vertebroplasty.15 Other studies reported restoration of mobility after kyphoplasty in 84% to 100%.8,16 In terms of other types of physical functioning, a number of different outcome measures have been used. In a retrospective analysis of patients with painful osteoporotic VCF, 49 patients who were available for follow-up at a mean 9-month interval had an improvement in visual analogue pain scale score of seven points (p < .05), and an improvement in Roland-Morris Disability Survey of 11 points (p < .05).17
In a retrospective analysis of 52 patients with 82 painful osteoporotic VCFs, kyphoplasty restored 4.6 mm and 3.9 mm to the heights of the anterior and medial columns, respectively.17 The mean Cobb angle increased by 14%. In a meta-analysis, Hadjipavlou et al concluded that, although postural reduction can improve vertebral height following a compression fracture, better reductions are obtained with kyphoplasty than with vertebroplasty.14 Better reductions may be achieved with earlier treatment.
Clinical Presentation and Evaluation
Successful kyphoplasty hinges on distinction of compression fracture pain from other etiologies. Clinical assessment involves an evaluation of the patient’s spinal alignment and gait, followed by palpation of the spine, ilium, sacrum, and paravertebral tissues. The importance of local tenderness over the involved spinous process as a principal sign of a painful VCF has been analyzed in two studies. In the first, which comprised 10 patients, Gaughen et al18 noted that local tenderness was not present despite imaging findings suggestive of an acute fracture. Recently, Gaitanis and coworkers16 found that spinous process tenderness corresponded to the level of pathology in 100% of osteolytic tumors and in 96% of VCFs when correlated with magnetic resonance imaging (MRI) findings of an acute fracture.
Several imaging techniques are employed in the evaluation of a painful VCF. Recently, flexion and extension or standing and supine lateral radiographs have been used to assess fracture mobility. A number of studies have examined the presence of intravertebral clefts. Although the exact cause of these intraosseous nitrogen pockets has been debated, the so-called Kummel sign may characterize pseudarthrosis. A cone-down lateral view directly perpendicular to the involved level is required in the assessment, because these clefts can easily be missed with standing lateral radiographs alone.19
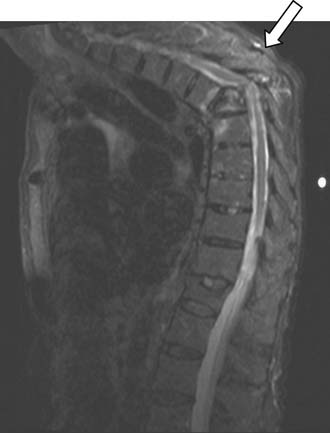
Magnetic resonance imaging is an important technique for detection of osteoporotic compression fractures (Figure 34-1). It is more sensitive than plain radiography, with a reported accuracy of 96%.19 Fracture acuity (or failure of healing) is also best observed as intense signal on sagittal MRI with short tau inversion recovery (STIR) sequences (Figures 34-2 and 34-3).20 For patients unable to undergo MRI, the combination of a technetium bone scan with computed tomography (CT) of the scintigraphically active levels can provide useful information on relatively fresh vertebral fractures (Figure 34-4).21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree