Figure 58.1. The chemical structure of LEV.
MECHANISM OF ACTION
Prior to undergoing standardized AED testing by the National Institutes of Health, LEV was found to have antiepileptic properties. In contrast to previously approved AEDs, LEV lacked conventional modulation of the acute seizure model (maximum electroshock seizure test and pentylenetetrazol), suggesting a novel mechanism of action (3–5). Moreover, LEV displays unique potent protection against kindled seizures in both mice and rats during kindling models (3,4). In comparative tests with established AEDs in a number of animal models of epileptic seizures, LEV displays potent protection in a broad range of animal models of chronic epilepsy, including partial and primary generalized seizures (5).
The mechanisms by which LEV exerts its AED effect are still not fully defined. Initial investigations of classic AED targets such as neuronal voltage-gated sodium, T-type calcium currents, or gamma-aminobutyric acid A receptors did not demonstrate sufficient action to account for the effectiveness of the drug (6,7). Subsequently, LEV was determined to have a unique central nervous system–binding site, synaptic vesicle protein 2A (SV2A) (8,9). SV2A is a component of secretory vesicle membranes and has been shown to mediate calcium-dependent vesicular neurotransmitter release (10–12). Binding affinity for SV2A has been shown to correlate with antiepileptic potency in animal models of audiogenic, partial, and generalized seizures (9,13). However, the mechanism by which binding of LEV to SV2A results in antiseizure activity is not yet known. This is a focus of ongoing research, given the potential importance of SV2A as a target for development of new antiepileptic agents.
ABSORPTION, DISTRIBUTION, AND METABOLISM
Overview
LEV is rapidly and almost completely absorbed following oral administration. The pharmacokinetics is linear and time invariant, with low individual variability (14). LEV is not protein bound (<10%), and its volume of distribution is close to the volume of intracellular and extracellular water (14,15). Sixty-six percent of the dose is unchanged as it is excreted renally (14). The major metabolic profile of LEV is an enzymatic hydrolysis of the acetamide group (14,15). LEV is not liver cytochrome P450 dependent (14). Its metabolites have no known pharmacologic activity and are renally excreted. The plasma half-life of LEV across studies is approximately 6 to 8 hours. The effects of the agent are increased in the elderly (primarily due to impaired renal clearance) and in patients with renal impairment (14,15).
Absorption and Distribution
Absorption of LEV is rapid, with peak plasma concentrations occurring about 1 hour following oral administration. Oral bioavailability is 100%, with no effect from ingestion of food. Linear pharmacokinetics characterizes LEV over a dose range of 500 to 5000 mg. Steady state is achieved after 2 days of multiple twice-daily dosing. LEV is <10% bound to plasma proteins; clinically significant interactions with other drugs through competition for protein-binding sites are unlikely (14,15).
Metabolism and Elimination
LEV is not extensively metabolized in humans with the major metabolic pathway of enzymatic hydrolysis of the acetamide group, which produces the pharmacologically inactive carboxylic acid metabolite. There is no dependency on P450 cytochrome liver metabolism (14,15).
LEV is eliminated by renal excretion as unchanged drug, which represents 66% of the administered dose (14). The total body clearance is 0.96 mL/min/kg, and the renal clearance is 0.6 mL/min/kg (14,15). The mechanism of excretion is glomerular filtration with subsequent partial tubular reabsorption. Elimination is correlated with creatinine clearance (CrCl) (14).
Special Populations
Pediatrics
The pharmacokinetics of LEV has been evaluated in children 6 to 12 years of age following single 20-mg/kg doses. The apparent clearance of LEV was approximately 40% higher in children than in adults. The half-life in children is 4 to 8 hours, compared with approximately 7 hours in adults. The maximum concentration of drug (Cmax) and area under the curve (AUC) values are comparable to those in adults. There is no correlation between age and clearance among pediatric patients (15).
Elderly
In older adults, total body clearance decreased by 38%, and the half-life was 2.5 hours longer compared with healthy adults (14).
Renal Impairment
Total body clearance of LEV is reduced in patients with impaired renal function by 40% in those with mild renal impairment (CrCl 50 to 80 mL/min), 50% in those with moderate impairment (CrCl 30 to 50 mL/min), and 60% in those with severe renal impairment (CrCl < 30 mL/min). In patients with end-stage renal disease, total body clearance decreased by 70% compared with those with normal renal function. About 50% of LEV is removed during a standard 4-hour hemodialysis procedure. Thus, dosage should be reduced in patients with impaired renal function, and supplemental doses should be given after hemodialysis (14).
Hepatic Impairment
The pharmacokinetics of LEV is unchanged in individuals with hepatic impairment. No dose adjustment is needed in patients with hepatic impairment (14).
DRUG INTERACTIONS
In vitro data on metabolic interactions indicate that LEV is unlikely to produce or be affected by pharmacokinetic interactions. Minimal plasma protein binding makes interactions due to competition for protein-binding sites unlikely (16). Potential pharmacokinetic interactions were assessed, but none were reported in clinical pharmacokinetic studies with phenytoin, warfarin, digoxin, and oral contraceptives (17–20). Analysis of Phase 3 studies also revealed no pharmacokinetic interactions with other AEDs, such as phenytoin, carbamazepine, valproic acid, and phenobarbital (17,21).
EFFICACY
Partial-Onset Seizures
The effectiveness of LEV as adjunctive therapy in adults was established in three multicenter, randomized, double-blind, placebo-controlled clinical trials in patients with refractory partial-onset seizures with or without secondary generalization (22–24). Patients (N = 904) were randomized to one of four treatment arms: placebo, LEV 1000 mg, LEV 2000 mg, or LEV 3000 mg/day. Responder rates (50% or more reduction in seizure frequency compared with baseline) of 37.1% and 20.8%, respectively, were reported for 1000-mg/day doses for studies 1 and 2. At 2000 mg/day, a responder rate of 35.2% was reported; responder rates of 39.6% and 39.4%, respectively, were noted for 3000 mg/day (22–24). All of the response rates were statistically significant when all three LEV treatment arms were compared with placebo. Complete seizure freedom was reported to be 2% at 1000 mg and 6.7% at 3000 mg/day (22–24).
An interesting finding in study 1 was the rapid onset of efficacy of LEV (22). A significant reduction in weekly seizure frequency compared with that of the baseline period was observed during the first 2 weeks of the titration period, indicating that the agent has a rapid clinical effect at an initial dose (22). Open-label community trials confirmed the results noted in the pivotal trials, with efficacy achieved in patients at a dose of only 500 mg b.i.d. (25).
Four published studies have demonstrated the sustained efficacy of LEV as add-on epilepsy therapy for a period of at least 12 months and for as long as 54 months. The long-term tolerability of the agent is similar to that seen in the short-term, placebo-controlled trials (26–31).
Myoclonic Seizures
The effectiveness of LEV as adjunctive therapy in patients 12 years of age or older with juvenile myoclonic epilepsy (JME) experiencing myoclonic seizures was established after one multicenter, randomized, double-blind, placebo-controlled study (32). One hundred twenty-two patients were randomized to either placebo or LEV at a target dose of 3000 mg/day. A total of 58.3% of the LEV group responded (>50% reduction from baseline in myoclonic seizure days per week) versus 23.3% of those in the placebo arm; 25% of those treated with LEV were seizure free.
Primary Generalized Tonic–Clonic Seizures
LEV was evaluated for efficacy as adjunctive therapy in patients with idiopathic generalized epilepsy experiencing primary generalized tonic–clonic seizures by one randomized controlled multicenter trial (33). One hundred sixty-four subjects aged 4 to 65 years were randomized to either placebo or a LEV dose of 3000 mg/day or 60 mg/kg/day for children, added to one or two baseline AEDs. 77.2% of the LEV-treated group had a >50% reduction in GTC frequency versus 45.2% in the placebo arm. More subjects in the LEV arm were also seizure free (34.2% vs. 10.7%) during the evaluation period.
Monotherapy
Individuals with refractory partial epilepsy who completed a multicenter, double-blind, placebo-controlled parallel group with LEV 3000 mg/day were eligible for a monotherapy trial (24). Forty-nine patients entered the monotherapy arm. The median percent reduction in partial seizures was 73.8%, with a 50% responder rate of 59.2%. Nine patients (18.4%) remained seizure free on monotherapy (24).
In a multicenter, noninferiority comparison trial conducted in newly diagnosed patients with epilepsy, LEV was compared to controlled-release carbamazepine as initial treatment. At 1 year, seizure outcomes were similar, with 56.6% of patients randomized to LEV (N = 288) and 58.5% of patients receiving controlled-release carbamazepine (N = 291) were seizure free. Withdrawal rates due to treatment-emergent adverse events were 14.4% with LEV and 19.2% for those treated with controlled-release carbamazepine (34). LEV is not currently FDA approved as initial monotherapy.
Pediatrics
LEV has been evaluated in partial-onset seizures in children with epilepsy. One randomized, double-blind, placebo-controlled study was performed in North America with 60 sites and 198 pediatric patients between the ages of 4 to 16 years of age (35). Patients were randomized to placebo or to a dose of 20 mg/kg/day in two divided doses to a target dose of 60 mg/kg/day. The results showed a responder rate of 44.6% and a 26.8% reduction in weekly partial-onset seizures. A comparative trial was recently performed comparing LEV versus carbamazepine monotherapy for partial epilepsy in children <16 years of age. LEV was shown to have equal efficacy to carbamazepine (36).
Two open-label trials have been conducted to assess the efficacy and safety of LEV in children with partial seizures (15,37). Twenty-three children 6 to 12 years of age with treatment-resistant partial-onset seizures who were receiving one standard AED were eligible (32). Seizure frequency in these children was evaluated and compared with a 4-week baseline seizure frequency, using a 6-week titration to a target dose of 40 mg/kg/day. Twelve children (52%) responded (50% seizure reduction), with two patients remaining seizure free during the entire study period (37).
A small randomized trial of LEV versus placebo for children and adolescents with newly diagnosed absence epilepsy found no statistically significant response with short-term use (38).
Status Epilepticus
The availability of an intravenous formulation of LEV that can be infused relatively quickly without hemodynamic side effect has led to multiple case series reported in the literature. Ten of the highest quality studies were identified and systematically reviewed, which included a total of 334 patients. Of the patients reported, 279 were treated with LEV after the administration of a benzodiazepine, while the remaining 55 were treated as initial therapy. The definition of efficacy varied between the reviewed studies and ranged between 44% and 94%. No serious adverse effects were reported. The most common side effect noted was somnolence in up to 40%. Caution should be used when attempting to generalize these data, since none of these studies were controlled trials and 8 of the 10 studies were retrospective. Also, due to the design of these studies, many of these patients had relative contraindications to traditional therapies (39).
Subsequent to the systematic review, a larger randomized prospective open label trial of 79 patients in convulsive status epilepticus was published comparing LEV 20 mg/kg infused over 15 minutes to lorazepam and 0.1 mg/kg over 2 to 3 minutes. Primary end point was the cessation of seizure activity by 30 minutes. There was no statistical difference between the two groups for first-line therapy (LEV 79.8% vs. lorazepam 78.2%). Also, there was no statistical difference in response when crossed over to second-line therapy, seizure recurrence in 24 hours, and seizure freedom at 24 hours (40). From these initial data, a prospective randomized multicenter trials are planned to determine the effectiveness of LEV and other therapies in benzodiazepine refractory status epilepticus (41). LEV is not currently FDA approved for the indication of status epilepticus.
ADVERSE EVENTS
Central Nervous System
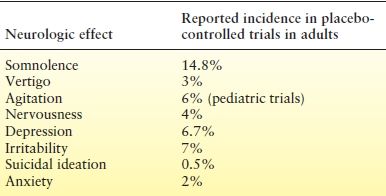
Three main types of CNS adverse effects are associated with LEV use: fatigue, coordination difficulties, and behavioral problems (22–24,37). In the three pivotal clinical trials, 14.7% of patients reported fatigue, whereas 3.4% had coordination problems. Coordination difficulties included ataxia, abnormal gait, and incoordination. Dose reduction improved these symptoms. Fatigue and coordination problems occurred most frequently within the first 4 weeks of treatment. Of patients treated with LEV, 13% reported such behavioral symptoms as agitation, hostility, anxiety, apathy, emotional lability, depersonalization, and depression (Table 58.1). Most of these symptoms occurred within 4 weeks of drug initiation (22–24). Dose reduction was associated with improvement in these behavioral problems, with only 0.8% of treated patients requiring hospitalization. In the open-label trial of children, there were no differences between adverse events reported in this population and those reported in adults (42).
Table 58.1 Neurologic and Psychiatric Adverse Effects of LEV
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








