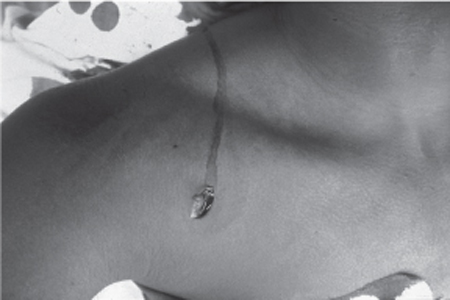
30 Management of Penetrating (Lacerating) Nerve Injuries A 17-year-old male was the victim of several stab wounds. The wound relevant to this discussion was located approximately 1 cm below the right clavicle, approximately at the medial border of the lateral third of the clavicle (Fig. 30–1). Neurological examination of the right upper extremity revealed at least grade 4 function of the deltoid muscle according to the Medical Research Council (MRC) scale. Elbow flexion, wrist flexion, and finger flexion were also at least grade 4. Elbow extension, wrist extension, and finger extension were absent. Wrist and finger flexion as well as intrinsic musculature of the hand were functional, indicating that the median and ulnar nerve were spared. The right upper extremity was well vascularized, with intact distal pulses. The patient was taken to the operating room with the tentative working diagnosis of a proximal (axillary level) right radial nerve laceration. At surgery, the stab wound was incorporated into a curvilinear incision allowing exposure of the infraclavicular brachial plexus. The axillary artery was identified and found to be uninjured. Also uninjured were the medial and lateral cords, which surrounded the artery in their usual anatomical location. The distal posterior cord was then identified and traced proximally until the zone of injury was located. The medial half of the posterior cord, specifically those fascicles giving rise to the radial nerve proper, had been sharply lacerated. The posterolateral components of the posterior cord, including the components destined to become the thoracodorsal, subscapular, and axillary nerves, were in continuity. Under the operating microscope, the ends of the lacerated nerve were sharply debrided, and a tension-free primary epineurial repair was performed using 8–0 nylon microsuture. At 6-month follow-up, the patient was found to have excellent and normal deltoid function. Radial nerve function continued to be absent, with no contraction of triceps, wrist, finger, and thumb extension. The patient will continue to be followed, and consideration may be given to tendon transfer procedures to partially restore thumb and finger extension and to improve extension at the wrist while awaiting recovery of his neuromuscular function. Penetrating injury of the brachial plexus with posterior cord (to radial nerve) laceration Figure 30–1 Preoperative photograph depicting the stab wound, which was located approximately 1 cm below the right clavicle, roughly at the medial border of the lateral third of the clavicle. The surgical incision incorporated the stab wound and allowed exposure of the infraclavicular brachial plexus. The cords of the brachial plexus are named according to their relationship to the axillary artery. In the axilla, the posterior cord gives rise to five branches: the upper and lower subscapular nerves (C5–6), thoracodorsal nerve (C6–8), axillary nerve (C5–6), and radial nerve (C5–T1). The topographic relationships of these branches are important to consider: the medial half of the posterior cord gives rise to the radial nerve proper (including a few branches that innervate the triceps), whereas the posterolateral components of the posterior cord include the components destined to become the thoracodorsal, subscapular, and axillary nerves. The upper and lower subscapular nerves are small nerves that arise from the proximal posterior cord and innervate the subscapularis muscle. The thoracodorsal nerve runs down the posterior axillary wall and enters the deep surface of the latissimus dorsi, which it terminally innervates. The axillary nerve, after arising from the posterior cord, courses posteriorly through the quadrangular space, which is bounded by the subscapularis above, the teres major below, the long head of the triceps medially, and the surgical neck of the humerus laterally. The radial nerve is the main continuation of the posterior cord. It crosses the inferior border of the posterior axillary wall, superficial to the tendon of the latissimus dorsi. The nerve passes obliquely across the back of the humerus from medial to lateral in the spiral grove. It then courses through the triangular space below the lower border of this tendon because it lies in front of the teres major and between the long head of the triceps and the humerus. The association of a sharply penetrating object with a neurological deficit is highly suggestive of a lacerating nerve injury. In approximately 85% of cases, the penetrating object will transect the nerve. In the remainder of cases, the nerve is bluntly contused. The diagnosis and localization of a plexus lesion are based on the clinical findings. In this case, the location of the laceration and the palsy affecting all aspects of radial nerve function, including elbow extension (triceps), while sparing the latissimus dorsi and deltoid, clearly localized the lesion to the very proximal radial nerve or the posterior cord contribution to radial. The goal of the immediate management of traumatic brachial plexus injuries is to differentiate between those injuries that will benefit from early surgical intervention and those injuries that should be followed expectantly. This differentiation is based upon determining the cause, site, and severity of the disruption in the plexal elements. In the vast majority of instances, a thorough history and physical examination will be sufficient to make many of the early management decisions and to formulate a tentative treatment plan. Implicit in this is a detailed working understanding of the anatomy of the brachial plexus. Although the history and physical examination are of utmost diagnostic importance in these cases, ancillary electrophysiological and radiological investigations may also be useful in certain select circumstances. In evaluating traumatic injuries to the brachial plexus, the key issue thus becomes differentiating between a closed and an open injury. Second, it is important to determine the immediate postinjury status of the plexus from a functional perspective, so that one can determine if the severity of the neurological deficit is changing over time. Clearly, nearly all patients will have their maximal loss immediately; if the deficit is worsening, then continued or increasing pressure on the nerves is implied. In the case of penetrating injuries to the brachial plexus, the differential diagnosis for progressive loss of function includes compression by a focal hematoma, or the development of an axillary artery pseudoaneurysm or arteriovenous fistula. In these circumstances, prompt imaging and surgical intervention are required to prevent permanent loss of function. The overall management decisions involved in treating penetrating injuries to the brachial plexus are far less complex than those required in the management of closed or traction injuries to the plexus. In the vast majority of open brachial plexus injuries, the injured plexal element can be assumed to be no longer in continuity. Thus the lesion may be classified as neurotmetic, and no recovery of function can reasonably be expected without primary surgical repair. Rather than deciding how long to wait to determine whether the neural element is in continuity, which is the key dilemma in evaluating closed injuries to the plexus, for penetrating trauma, the decision simply lies in determining the proper time frame in which to intervene. Early exploration and repair constitute the gold standard for managing penetrating trauma to the brachial plexus. When the laceration is clean and sharp, the operative repair should be done as soon as possible and ideally within the first 48 to 72 hours postinjury, although there is no indication to treat this as a surgical emergency. Nerves may also be bluntly transected by other implements, such as industrial machinery, bone, and saws. These implements produce injuries that are different from clean, sharp lacerations in that they produce significantly more local soft tissue damage in the vicinity of the injured nerve. The component of blunt trauma to the nerve, in the form of stretch and contusion of the fascicles, may be significant. Moreover, there is a higher incidence of associated fractures and vascular tears, which may need to be addressed prior to the repair of the plexus itself. The management of open injuries produced by these objects differs somewhat from the principles employed in managing clean, sharp lacerations to the plexus, such as those produced by a knife or a piece of broken glass. If a wound is jagged, torn, contused, or otherwise contaminated, it is best to delay repair of the neural elements until the wound is clean. The gross contaminated wound is therefore debrided and irrigated and closed following general principles of wound surgery. With respect to the neural repair itself, one must keep in mind that the nerve will almost certainly be damaged well beyond the point of actual division. The extent of actual injury cannot be easily discerned by visual inspection. Therefore, in this circumstance, it is best to delay the nerve repair for 3 to 4 weeks to let the nerve injury demarcate. Once the nerve demarcates, the proximal and distal neuromas can be resected back to areas of normal fascicular architecture, and the repair can then be carried using nerve grafts as discussed following here. In complex open injuries, if the wound is to be explored acutely for other indications, such as vascular repair or debridement, then it is prudent to identify the nerve ends and tack them down to the surrounding connective tissue and fascia (using nonreabsorbable large-caliber sutures) at this time. Tacking down the nerve ends at the time of the initial operation serves two important functions: it allows easier identification of the injured nerve at the time of reoperation, and limits the shortening of the nerve due to retraction. An interposition nerve graft is often needed for the delayed repair of a penetrating brachial plexus injury. The basic principles are similar to those underlying the end-to-end epineurial repair. The gap is measured, and 10% is added to the gap to determine the approximate length of the graft required. The sural or antebrachial cutaneous nerves are preferred because multiple small-caliber grafts give better results than larger-caliber grafts. No convincing differences have been demonstrated between vascularized and nonvascularized grafts. A special subset of penetrating injuries to the brachial plexus are those caused by high-velocity missiles (i.e., gunshot wounds). Gunshot wounds produce complex, concussive, “blast” effects on the surrounding connective tissue. In many instances, the neural elements are severely damaged and produce complete deficits but remain in continuity. In fact, less than 10% of gunshot wounds to the brachial plexus produce neurotmetic lesions. Because most gunshot wounds leave the injured nerve in continuity, a conservative approach, such as that employed in managing stretch injuries to the brachial plexus, is the favored management strategy. A period of observation, usually of 3 to 4 months, is the usual initial treatment, aided by baseline electromyography, and nerve conduction studies performed at approximately 4 weeks. If there is no evidence of clinical or electrophysiological recovery after this interval, then surgical exploration is warranted. At exploration, adjunctive use of nerve action potential recording is useful to determine for resection of the nonconducting neuroma. Analyzing the results of brachial plexus repair in general, and for penetrating injuries in particular, is a difficult task owing primarily to the heterogeneity in the documentation of outcome measures. Furthermore, many of these injuries are complex, with significant overlap of different mechanisms, each of which has the potential to ultimately affect the patient’s outcome. What can be said with relative certainty, however, is that the acute primary repair of a sharply lacerated nerve offers the highest probability of successful innervation of distal musculature. Primary end-to-end repairs generally yield better results than an interposition nerve graft repair. This reality is a simple function of the fact that regenerating axons must cross two suture lines in a graft repair, rather than a single one when an end-to-end coaptation is undertaken. Given these principles, repair of sharply transected nerves is often associated with somewhat better outcomes than the delayed repair of bluntly transected nerves. Of all penetrating lesions, gunshot wounds to the brachial plexus carry the worst prognosis. Penetrating injuries to the brachial plexus can be distinguished from closed, traction-type injuries on the basis of a thorough history and physical examination. This distinction is vital because penetrating injuries have a different management paradigm from that employed in treating closed injuries. There is some heterogeneity in the management strategies for penetrating brachial plexus trauma because different sharp implements produce different effects on the neural elements. For clean, sharp lacerations, such as those produced by a knife, glass, or scalpel, a primary end-to-end epineurial repair performed within the first 48 to 72 hours after injury is the preferred treatment option. For other implements, which produce not only a nerve laceration but also a variable degree of blunt trauma to the neural elements as well, it is prudent to delay exploration and repair for 3 to 4 weeks to allow damaged areas of the nerve to demarcate clearly. After debriding back to healthy fascicular tissue, if tension-free end-to-end repair is not possible, then a nerve graft is employed. Nerve grafts similarly have to be employed in the delayed repair of initially sharply divided nerve elements because the ends retract significantly over time. Finally, for gunshot wounds to the plexus, the management scheme is similar to that for closed injuries because fewer than 10% of these injuries result in actual division of the nerve.
 Case Presentation
Case Presentation
 Diagnosis
Diagnosis
 Anatomy
Anatomy
 Characteristic Clinical Presentation
Characteristic Clinical Presentation
 Differential Diagnosis
Differential Diagnosis
 Management Options
Management Options
 Outcome and Prognosis
Outcome and Prognosis
 Conclusions
Conclusions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


