Chapter 220 Management of Symptomatic Osteoporotic Vertebral Compression
Vertebroplasty
Vertebral Augmentation
Kyphoplasty and vertebroplasty currently are used to treat neurologically intact patients suffering from vertebral compression fractures resulting from osteoporosis and certain neoplasms. Percutaneous vertebroplasty (PV) is an imaging-guided procedure that reinforces a compromised vertebra with polymethylmethacrylate (PMMA), alleviating pain and improving the patient’s mobility. Initially described in 1987 as a treatment for painful hemangiomas,1 the procedure now is used most widely to treat fractures and destructive lesions that cause vertebral collapse and pain. The goal of vertebral augmentation procedures is to reduce fracture pain by stabilizing the vertebral fracture, allowing patients to return to their daily living activities and exercise.
The primary indications for PV are persistently painful compression fractures, most commonly related to osteoporosis, that are unresponsive to correct medical treatment, and benign or malignant osteolytic neoplasms (e.g., hemangioma, metastasis, and myeloma). In the setting of osteoporotic fracture, PV is done primarily for pain management and secondarily to prevent further collapse.2 For neoplasm, the indications are somewhat broader, and the procedure may be done for pain management and/or stabilization. PV is not an ablative procedure, but reinforcement may reduce pain while facilitating other therapies such as radiation therapy or surgical resection and fixation and minimizing the risk of further collapse or fracture.
The specific indications for PV are as follows:
• Osteoporotic compression fractures causing pain refractory to nonsurgical therapy and interfering with normal activities of daily living
• Multiple compression deformities resulting in, or threatening to result in, respiratory or gastrointestinal compromise or loss of balance, increasing fall risk
• Unstable compression fractures showing movement or progressive collapse
• Osteolysis due to malignant or benign neoplasms with fracture or impending risk of fracture
Relative contraindications include the following:
• An asymptomatic and stable fracture
• A patient showing symptomatic improvement with time
• Prophylactic vertebral augmentation in the absence of any acute compression fracture
Other, less common, relative contraindications are (1) retropulsion of fracture fragment(s) causing substantial spinal canal compromise (e.g., a burst-type fracture); (2) neoplasm extending into the epidural space with substantial spinal canal compromise; (3) severe vertebral collapse such that it would be technically challenging to place a needle; (4) chronic stable fracture without pain; and (5) treatment of more than three levels at any one time. Acute traumatic fracture of a nonosteoporotic vertebra also is considered a contraindication.
Technical Performance of Vertebroplasty
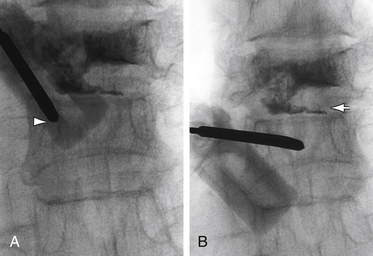
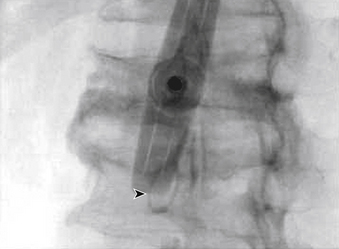
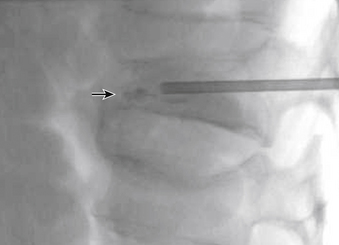
The traditional mode for PV has been to perform bilateral injections via a transpedicular or dorsolateral (parapedicular) approach using a two-needle technique (Figs. 220-1 to 220-5). Unipedicular injections also can provide substantial vertebral reinforcement when enough cement is injected to cross the midline of the vertebral body and provide a similar distribution to bipedicular injections.3
The modalities used for imaging are either fluoroscopy or CT. PV can be accomplished with either single-plane or biplane fluoroscopy. Biplane fluoroscopy is considered ideal because one can view orthogonal projections without changing tube position, greatly reducing procedure time. CT requires repositioning the gantry between needle manipulations, imaging, and injection, consuming useful injection. CT fluoroscopy allows the user to generate real-time CT sections while performing procedures, but concerns with CT fluoroscopy revolve around the increased radiation dose delivered to personnel and the operator in the room during the procedure. For most purposes, C-arm fluoroscopy provides adequate imaging, is readily available, and is less time-consuming.
Cement Handling
The two most popular preparations used for PV are Cranioplastic Type I Slow Set (Codman, Johnson & Johnson) and Simplex P (Stryker-Howmedica-Osteonics). Simplex P is approved by the FDA as a structural device for use in pathologic fractures in bones throughout the body, but the approval does not specify PV per se. Simplex P was the original PMMA used for the first PV by Deramond in 1984 and has remained popular for this application in Europe and the United States. In a comparison of three types of PMMA in cadavers (Cranioplastic, Osteobond, and Simplex P), vertebrae were significantly stronger after cement injection, regardless of cement type. However, Simplex P restored stiffness to initial values, whereas vertebrae injected with Cranioplastic were significantly less stiff than in their initial state.4
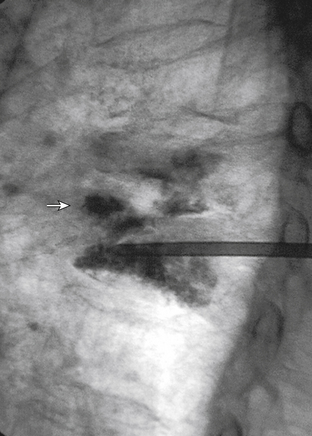
These preparations (mixed according to the package insert) produce cement that is difficult to inject and poorly visualized by fluoroscopy (although quite suitable for radiography). Thus the addition of an opaque agent (sterile barium, tantalum, or tungsten) is required. Sterile barium preparations are available from Parallax (Mountain View, CA) or Bryan Corporation (Woburn, MA). It has been determined that PMMA mixtures containing approximately 25% to 30% by weight of barium sulfate will provide opacification sufficient for the performance of fluoroscopically guided PV.4,5
With respect to how much cement mixture to inject, an in vitro study demonstrated that initial vertebral body strength is restored with as little as 2 mL of cement, but significantly greater stiffness requires 4 to 8 mL, depending on vertebral level and type of cement.6 These data provide guidance on the cement volumes needed to restore biomechanical integrity and parallel the clinical experience that many patients do well without having the cement fill an entire vertebral body. Hence, the trend has been to use less cement, minimizing the risk of complications from extravasation.
New biomaterials such as nanoparticles and Orthocomp (a glass-ceramic-reinforced BisGMA/BisEMA/TEGDMA matrix composite) are being actively pursued, and it is likely that specific formulations will be available for PV in the near future.7
Treating Tumors
Vertebroplasty may play an important role in palliation for the patient with vertebral metastases and in improving independence and function during more definitive systemic therapy. Tumors that are particularly radiosensitive are most appropriate to PV, because local control can be obtained as easily after stabilization as before. Tumors that require resection with a surgical margin, such as primary malignancies or locally aggressive benign tumors, are not appropriate for PV. Although there is some theoretical margin of tumor-kill associated with the thermal effect of the PMMA mass,8 the application of PV in the setting of neoplasm is not intended as an ablative procedure, and the goal is to provide structural support primarily, with the secondary goal of pain relief. The amount of PMMA used may be greater in an osteolytic spinal lesion than in a compression fracture, because the trend to minimize injectant volume in osteoporosis (i.e., filling the fracture line) does not apply in an erosive or destructive lesion.
Metastases are the lesions most commonly treated by PV, but large, symptomatic hemangiomas also may require ablation and stabilization with PMMA injection. Hemangiomas have a benign histology, but may grow aggressively and cause pain through either tissue distortion or pathologic fracture. PV stabilizes the vertebral body and obliterates the vascular sinusoids that make up the mass of the hemangioma. Subsequent surgery may then be focused on decompression, if needed.9 In this fashion, PV is another adjuvant treatment akin to intralesional sclerosis or embolotherapy preoperatively. PV also is effective in treating vertebral metastases that result in pain or instability, providing immediate and long-term pain relief.10 MRI is crucial for preoperative planning to evaluate the soft tissue extent of the tumor (e.g., spinal canal involvement, neuroforaminal encroachment, and status of the posterior longitudinal ligament).
Complications
Fortunately, major complications are uncommon. The most common complication is radicular pain caused by migration of cement into the epidural venous plexus. In most patients, intradiscal and paravertebral leaks of cement have no clinical importance.11 However, there have been case reports of severe neurologic complications, underscoring the need for appropriate safeguards as outlined previously.12 Permanent paralysis has been reported, but is exceptionally uncommon if the procedure is performed in a controlled, image-guided fashion (by using a biplane real-time fluoroscopy suite). Rib, pedicle, or transverse process fractures also have been noted. One case has been reported of a pulmonary embolism caused by acrylic cement. This rare complication was believed to have occurred because perivertebral venous migration was not recognized.13 The anticipated complication rate is higher when treating neoplasms (10%) than for osteoporotic compression fractures (1% to 3%).
Outcomes
Taylor et al. performed a systematic review and meta-regression to compare the efficacy and safety of balloon kyphoplasty and vertebroplasty for the treatment of vertebral compression fractures, and to examine the prognostic factors that predict outcome. They found level III evidence to support balloon kyphoplasty and vertebroplasty as effective therapies in the management of patients with symptomatic osteoporotic vertebral compression fractures refractory to conventional medical therapy. Although there was a good ratio of benefit to harm for both procedures, balloon kyphoplasty appeared to offer the better adverse event profile.14
In a follow-up study, the same authors concluded that in direct comparison to conventional medical management, patients undergoing kyphoplasty experienced superior improvements in pain, functionality, vertebral height, and kyphotic angle, at least up to 3 years postprocedure. Reductions in pain with kyphoplasty appeared to be greatest in patients with newer fractures. The authors concluded that there are prospective studies of low bias, with follow-up of 12 months or more, that demonstrate balloon kyphoplasty to be more effective than medical management of osteoporotic vertebral compression fractures and at least as effective as vertebroplasty.15
Kallmes et al. randomly assigned 131 patients who had one to three painful osteoporotic vertebral compression fractures to undergo either vertebroplasty or a simulated procedure without cement injection (served as control group).16 The primary outcomes were scores on the modified Roland-Morris Disability Questionnaire (RDQ) and patients’ ratings of average pain intensity during the preceding 24 hours at 1 month. Patients were allowed to cross over to the other study group after 1 month. Interestingly, both groups had immediate improvement in disability and pain scores after the intervention. At 1 month, there was no significant difference between the vertebroplasty group and the control group in either the RDQ score or the pain rating. The authors found a trend toward a higher rate of clinically meaningful improvement in pain (a 30% decrease from baseline) in the vertebroplasty group at 1 month. At 3 months, there was a higher crossover rate in the control group than in the vertebroplasty group. There was one serious adverse event in each group. The authors concluded that improvements in pain and pain-related disability associated with osteoporotic compression fractures in patients treated with vertebroplasty were similar to the improvements in a control group.
In a prospective study of 30 consecutive patients, Muijs et al. analyzed clinical and radiologic outcome 36 months after percutaneous vertebroplasty for osteoporotic vertebral compression fractures unresponsive to conservative treatment for at least 8 weeks. The authors also examined the quality of life (QOL). The authors reported good pain relief and significant increase in QOL scores, despite finding asymptomatic leakage of cement in 47 of 58 (81%) of treated vertebrae. The authors concluded that percutaneous vertebroplasty in the treatment of chronic vertebral compression fractures results in an immediate, significant, and lasting reduction in back pain, as well as overall improvement in physical and mental health.17
A meta-analysis to study the amount of pain reduction using the visual analog scale (VAS) with kyphoplasty and vertebroplasty in the treatment of osteoporotic vertebral compression fractures revealed that both procedures reduce pain in symptomatic osteoporotic vertebral compression fractures that have failed conservative treatment.18
Pain relief and risk of complications associated with vertebroplasty versus kyphoplasty were evaluated by Eck et al.19 The authors identified a total of 1036 abstracts for potential inclusion. Of these, 168 studies met the inclusion criteria. Mean preoperative and postoperative VAS scores for vertebroplasty were 8.36 and 2.68, respectively, with a mean change of 5.68 (P < .001). The mean preoperative and postoperative VAS scores for kyphoplasty were 8.06 and 3.46, respectively, with a mean change of 4.60 (P < .001). Statistically greater improvement was found with vertebroplasty versus kyphoplasty (P < .001). The risk of new fracture was 17.9% with vertebroplasty versus 14.1% with kyphoplasty (P < .01). The risk of cement leak was 19.7% with vertebroplasty versus 7.0% with kyphoplasty (P < .001). The authors concluded that vertebroplasty had a significantly greater improvement in pain scores but also had statistically greater risk of cement leakage and new fracture.
Outcomes in Patients with Vertebral Compression Fractures
Buchbinder et al. performed a multicenter, randomized, double-blind, placebo-controlled trial. Participants had one or two painful osteoporotic vertebral fractures that were of less than 12 months’ duration and unhealed. Participants were randomly assigned to undergo either vertebroplasty or a sham procedure.20 The primary outcome was overall pain at 3 months. These authors found no beneficial effect of vertebroplasty as compared with a sham procedure in patients with painful osteoporotic vertebral fractures, at 1 week or at 1, 3, or 6 months after treatment.
Masala et al. reviewed 624 patients with 1253 compression fractures that were treated by percutaneous vertebroplasty and found a statistically significant improvement in the patients’ quality of life by 12 months.21 In a separate retrospective study, Masala et al. evaluated the effectiveness, costs, and cost-effectiveness of percutaneous vertebroplasty.22 After 2 weeks of analgesic therapy, 153 patients presented with refractory pain and were offered treatment by percutaneous vertebroplasty. A total of 58 patients accepted and underwent percutaneous vertebroplasty, while 95 refused and underwent conservative medical therapy. Significant reduction in VAS and improvement in ambulation and activities of daily living were observed in both groups at 1 week, 3 months, and 12 months. These results were significantly superior in the percutaneous vertebroplasty group at 1 week and 3 months. Percutaneous vertebroplasty was significantly more cost-effective than medical therapy with regard to VAS and activities of daily living at 1 week. By 3 months, percutaneous vertebroplasty was more cost-effective than medical therapy with regard to ambulation. No significant difference in cost-effectiveness was found between the two groups at 12 months. The authors concluded that percutaneous vertebroplasty should be considered the treatment of first choice in symptomatic acute osteoporotic vertebral fractures with refractory pain after a short period of analgesic therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree