Fig. 7.1
Clinical specimens from mechanical thrombectomy in acute stroke demonstrating: (a) the fibrin rich clot or so-called white clot, and (b) the red blood cell rich clot, termed red clot
In addition to clot composition, the initial clot length, or clot burden, on admission imaging is seen to play a role in revascularization and outcomes. In a seminal study by Riedel and colleagues [10], 138 consecutive patients with middle cerebral artery occlusion were studied who were treated with intravenous thrombolysis. All patients in the study had thin-slice noncontrast head CTs to measure the clot length as a measure of clot burden. Of these patients, 62 patients (45 %) demonstrated revascularization with the intravenous therapy alone. On further analysis, none of the patients who revascularized had clot length greater than 8 mm, indicating in this study group that a clot length of more than 8 mm in the middle cerebral artery was too great of a clot burden for intravenous therapy to be successful in treating. This clinical pattern of patients with large clot burdens being refractory to intravenous lytics, has provided a strong impetus for the development of endovascular mechanical devices designed for arterial revascularization in acute ischemic stroke.
Early Endovascular Devices
Thus far, endovascular mechanical thrombectomy devices have been primarily designed for thrombectomy of the large proximal arteries (ICA, M1/M2, Vertebral, and Basilar). This has been based on two primary factors: (1) thrombectomy devices appear to be safest and most effective in 2–5 mm vessels, and (2) either intravenous or intraarterial tPA appear to work well in the smaller clot burdens of the smaller vessels. In the early experience with intra-arterial lytic infusion for acute ischemic stroke, it was noted that passing the microcatheter through the clot and other mechanical manipulation of the clot assisted in breaking up the clot and assisted in clot dissolution. Prior to the development of dedicated clot retrieval devices, some of the early tools for mechanically disrupting clot included the use of intra-arterial angioplasty balloons and foreign body endovascular snare or retrieval devices [11]. Likewise, the development of the EKOS catheter (EKOS Corporation, Bothell, WA), an ultrasound accelerated thrombolytic microcatheter, increased the disruption of the clot to assist the lytic agents in revascularization [12]. As these early devices were not designed to retrieve clots, however, they often resulted in breaking up the clot, which resulted in distal emboli. Similarly, as these devices were not specifically designed for cerebral artery revascularization, they could lead to mechanically induced spasm of the artery, or arterial dissection.
The first device that received 510 k clearance by the Food and Drug Administration (FDA) for clot retrieval for cerebral artery emboli was the MERCI device, pioneered by Concentric Medical (Mountain View, CA) in 2004. The device was cleared specifically as a clot retrieval device, not approved as a stroke therapy device, as its safety approval trial demonstrated effective revascularization of cerebral arteries, but did not establish a therapeutic outcome improvement measure in acute stroke therapy since, as a single arm study, there was no control group. The initial iteration of the MERCI device was a bare metal corkscrew-shaped nitinol wire which could be delivered through a microcatheter with a 0.021 in. inner lumen. The microcatheter was advanced beyond the clot with a microwire for guidance, then the coiled device was unsheathed within the clot to engage it and pull it out. The phase 1 safety trial [13] in 28 patients evaluated patients who were within 8 h of stroke onset, had an admission NIHSS ≥ 10, had large vessel occlusion confirmed on imaging, had no large hypodensity (greater than 1/3 of the MCA territory) on admission head CT imaging, and had a contra-indication to intravenous tPA therapy. The mean admission NIHSS was 22, indicating a very severly affected patient group with large vessel occlusion. The successful TIMI (thrombolysis in myocardial infarction) revascularization rate (judged as TIMI 2 or TIMI 3) was 43 % with the MERCI device alone, and 64 % with the addition of intra-arterial tPA. Regarding clinical outcomes, 50 % (9/18) of patients revascularized had a good outcome (mRS 0–2) versus none (0/10) of the patients who were not revascularized had a good outcome. Although, there wasn’t a non-treatment control group, the interventional treatment group who could not be revascularized confirmed a poor outcome in patients with large vessel occlusion who could not be revascularized, indicating that, if the device worked, better clinical outcomes were seen.
The use of a proximal flow-arresting balloon guide catheter with this system, increased the efficacy of the procedure, by decreasing clot embolization to other branch vessels or distally as the clot was being extracted. The second generation of this device changed the coil memory of the nitinol to a cylindrical helix with attached prolene filaments to better ensnare the clot on retrieval (Fig. 7.2). The Multi-MERCI trial [14] demonstrated an improved revascularization rate with these device improvements. While the revascularization of the first generation device in the Multi-MERCI trial was 48 % without any adjuvant lytic, the secondary generation device demonstrated a 57 % revascularization rate without lytic. Still, even with these technical improvements, the time to revascularization remained substantial.


Fig. 7.2
The corkscrew shaped Merci device with prolene suture filaments attached in an attempt to better ensnare or engage the clot
The early corkscrew type retrieval devices encountered many challenges. In the early experience, multiple passes of the device were necessary to obtain revascularization, on average, just over three passes. In very recalcitrant clots, the clots were so firmly seated in the artery that the device coil memory would straighten with the resistance of the clot, or only a piece of the clot would be retrieved similar to a portion of a cork being removed from a bottle. Likewise, if there were underlying intracranial atherosclerotic disease in the occluded vessel, the coil memory would straighten in the stenotic segment, making it difficult for the device to fully engage the clot. Nevertheless, these early iterations of the device obtained quicker and more efficient revascularization in the acutely occluded arteries than intra-arterial tPA administration; provided a real option for patients who were not eligible for intravenous thrombolytics; and inspired a new generation of clot retrieval systems for acute stroke. In 2006, the Centers for Medicare and Medicaid Services (CMS) approved a new ICD-9 procedure code for mechanical thrombectomy with the MERCI device in the setting of acute ischemic stroke and began to cover this procedure.
Aspiration Catheters
In 2007, Penumbra, Inc. (Alameda, CA) received FDA 510 k clearance for the Penumbra System for clot retrieval in acute stroke [15, 16]. This system initially involved a series of variable diameter aspiration catheters ranging from 0.026 to 0.054 in. in inner luminal diameter, to be used in coordination with a beaded separator microwire, and aspiration system. The Penumbra Pivotal safety trial [16], for which the company received FDA clearance of the device, evaluated 125 patients in a single arm trial with NIHSS ≥8 within 8 h of onset of stroke, who were either ineligible for intravenous tPA or unresponsive to it. The study demonstrated an 82 % recanalization rate of TIMI 2 or 3, and clinical outcomes demonstrating a 25 % good outcome rate of modified Rankin score two or less. Further development of this technology led to improvements in aspiration catheter technology and improvements in the aspiration pump itself. An additional option, later made available for clot retrieval, was the clearance of a clot retrieval device shaped like a stent, known as the 3D device. This adjunctive device is currently under clinical trial.
The advent of the improved aspiration catheter design has made the direct aspiration of the clot a more realistic goal, without the use of a separator device or stent retriever [17]. This technique of direct aspiration of the clot without the use of a separator device, stent retriever, or microwire to disrupt the clot has been termed the ADAPT (ADirect Aspiration, first Pass Technique) technique. The benefits of this technique, when practical, are the very short clot extraction time, with potential groin puncture to revascularization times less than 10 min, and reduced risk of arterial injury, as the device or access wire never are passed blindly through the clot as is needed in all other clot retriever technology (Fig. 7.3). Additionally, if only an aspiration catheter is used, this is one of the more cost effective thrombectomy methods.
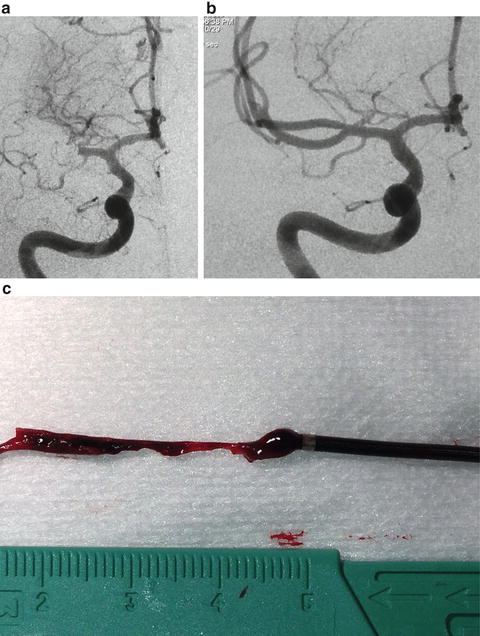

Fig. 7.3
(a) Pre-procedure right internal carotid artery angiogram in a patient with a right MCA occlusion who was 2 h from onset of stroke symptoms but not eligible for intravenous tPA. The admission NIHSS was 14. (b) Post-procedure right internal carotid artery angiogram image following mechanical thrombectomy with the ADAPT technique of direct clot aspiration. The total groin puncture time to TICI-3 revascularization was 6 min. The patient’s discharge mRS was 0. (c) Image of the clot lodged in the distal end of the aspiration catheter after retrieval from the middle cerebral artery
Stent Retrievers
2012 heralded the FDA clearance of the first two stent retrievers, the Solitaire FR (Covidien, Irvine, CA) and the Trevo (Stryker Neurovascular, Mountain View, CA). Both devices received FDA 510 k clearance demonstrating efficacy in clot retrieval equivalent, in fact superior, to the MERCI device. The basic technique and principal of the stent retriever technology is that a microcatheter is directed under roadmap guidance through the occlusive clot with a microwire for guidance, then the microwire is removed and the constrained, self-expanding, nitinol stent-shaped retriever is unsheathed within the clot. Since nitinol stent is self-expanding, the device traps the clot between the stent retriever and the artery wall, and, to varying degrees, insinuates the clot within the tynes of the stent, so that it may be successfully extracted. The stent is typically kept in its deployed position for a few minutes to allow the device to engage the clot prior to removal. In the majority of cases, keeping the stent open during this period allows for at least partial perfusion of the distal territory, and intra-procedural angiography with the stent retriever in place shows anterograde contrast flow through the prior point of clot occlusion, even prior to the clot retrieval.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





