Fig. 16.1
Schematic drawing of the inferior medullary velum and its relationship with the fourth ventricle and cisterna magna

Fig. 16.2
Schematic drawing of the tela choroidea (a) and inferior medullary velum (b), following spatulation of the cerebellar tonsils
The remaining 15 % present as a lateral mass, located in the cerebellar hemisphere or in the cerebellopontine angle. They may also involve the cerebellar surface, resembling meningiomas (Fig. 16.3). Non-midline tumors present predominantly in older children and adults [4].


Fig. 16.3
Sagittal (a), coronal (b), and axial (c) T1 contrast-enhanced MRI showing a medulloblastoma of the tentorial notch
Any patients presenting with a cerebellar tumor are candidate to surgery. If the patient is stable, surgery can be scheduled for the next available operating time. Usually surgery can wait for appropriate imaging studies, particularly brain and spinal MRI. Sagittal MRI can help delineate relationship of the tumor to the vermis, midbrain tectum, vein of Galen, and cervicomedullary junction and distinguish between true intraventricular tumor such as medulloblastomas that dislocate postero-superiorly the superior medullary velum and the quadrigeminal plate and widen the aqueduct [5] from vermian tumors such as astrocytomas, in which the quadrigeminal plate is dislocated anterior-inferiorly (Fig. 16.4). Because of high frequency of craniospinal metastasis, even at presentation, all the neuraxis should be mandatorily scanned before surgery.


Fig. 16.4
MR images of typical medulloblastoma arising from the inferior medullary velum, filling the fourth ventricle. Sagittal and coronal contrast-enhanced T1 WI images (a, b); Midsagittal DRIVE sequence (c)
However, if the patient develops acute hydrocephalus and mental status or vital sign changes, it may be necessary to treat hydrocephalus on urgent basis [6].
16.2 Management of Hydrocephalus
Hydrocephalus is a common presenting feature of medulloblastoma (about 80 % of cases), because of obstruction of egress foramina of the fourth ventricle. In about one third of patients, hydrocephalus persists following tumor removal [7].
Management of hydrocephalus in posterior fossa tumors is controversial. The choice between the available options (steroids and early surgery; external ventricular drainage (EVD); placement of ventriculoperitoneal (VP) shunt; endoscopic third ventriculostomy (ETV)) is usually left to the surgeon’s preference.
ETV has been often utilized in the management of hydrocephalus associated with posterior fossa tumors, since the publication of the study of Sainte-Rose et al. in 2001 [8]. These authors observed definitive treatment of hydrocephalus, improvement of the patient’s general condition, and improvement of postoperative course in most of their patients (94 %). Subsequent studies failed to confirm these very good results, questioning, above all, the possibility of ETV to provide long-term control of hydrocephalus [9–11]. Moreover due to the low incidence of persistent hydrocephalus following early tumor removal in some more recent series [9,10], many authors believe that the routine use of preoperative ETV is not entirely justified.
Medulloblastomas usually fall in the high-risk category for development of postoperative hydrocephalus: malignant histology, midline location, more severe ventricular enlargement at diagnosis, and younger age [12,13]. Patients with severe symptoms at presentation are also particularly at risk of a worse postoperative course [10].
In our centre, patients with midline tumors with severe hydrocephalus are considered candidate for preoperative ETV, rather than urgent posterior fossa surgery or external ventricular drainage. Tumor surgery is scheduled in the first available surgical session, following conclusion of the diagnostic workup and improvement of signs and symptoms of increased intracranial hypertension.
ETV eliminates the risks of CSF infection related to EVD and, providing more physiological CSF drainage than the other procedures, minimizes the risk of overdrainage. Placement of an EVD in these patients, in fact, must be exercised with caution because there is potential for upward herniation from posterior fossa mass effect.
El-Ghandour [14] reported the first study specifically addressed to midline posterior fossa tumors (medulloblastomas and ependymomas) in pediatric patients with advanced hydrocephalus. He compared pre-resectional ETV versus ventriculoperitoneal (VP) shunt. He concluded that the lower incidence of morbidity, the absence of mortality, the lower incidence of procedure failure of ETV as compared to VP shunt, and the significant advantage of not becoming shunt dependent makes endoscopic third ventriculostomy to be recommended as the first choice in the treatment of pediatric patients with marked obstructive hydrocephalus due to midline posterior fossa tumors.
In our experience, in very young babies (under 6 months of age) harboring tumors with radiological features of medulloblastoma, the results of ETV were disappointing. In fact almost all children required ventriculoperitoneal (VP) shunt in the postoperative period in spite of complete tumor resection. Our current policy in case of very young children (under 6 months of age) with acute hydrocephalus and midline posterior fossa tumor with radiological features of malignancy is to offer VP shunt urgently. In metastatic medulloblastoma at presentation, the patient should be referred to chemotherapy and radiotherapy as soon as possible. In this situation, endoscopic approach may be very useful to treat hydrocephalus, if associated, and to biopsy intraventricular lesion. The patient can be referred to oncologists before posterior fossa surgery [15] (Fig. 16.5).


Fig. 16.5
Brain (a) and spinal (b) MRI of metastatic medulloblastoma at presentation. The patient presented with acute hydrocephalus and was managed with insertion of ventriculoperitoneal shunt and endoscopic inspection of the ventricles, through a standard right precoronal burr hole. A small ependymal lesion was found at the level of the floor of the third ventricle that was biopsied. Histological diagnosis was medulloblastoma. The patient was referred to oncologists for chemotherapy and radiotherapy
In rare cases during endoscopic surgery, unrecognized metastases can be detected, especially at the level of the infundibulum (Fig. 16.6). Biopsy-confirmed metastases may change the staging of the tumor, switching from standard risk to high risk.


Fig. 16.6
Contrast-enhanced T1 WI MR images (a) and T2 MR images (b) of patient with a medulloblastoma, with no evidence of intraventricular metastases. (c, d): endoscopic view of the third ventricular floor covered by several small nodules. Histology confirmed the presence of malignant cells
In the management of postoperative hydrocephalus, ETV should be consider a valid alternative to shunt as a first option. In fact, there is agreement in the neurosurgical community in considering postoperative hydrocephalus obstructive in nature and to offer ETV to such patients [8–10].
Tamburrini et al. [16] have proposed a different strategy for management of hydrocephalus in posterior fossa tumors: perioperative external ventricular drainage positioned at time of tumor removal; postoperative ICP monitoring through the external ventricular drainage, ETV in case of persistent ventricular dilation and abnormally high ICP values, and VP shunt implantation in case of ETV failure.
16.3 Anesthetic Considerations
Care must be taken to avoid respiratory depression in all phases of the diagnostic and therapeutic workup, especially during sedation to obtain imaging and at the induction of anesthesia: even mild respiratory depression can cause dangerous increase in intracranial hypertension. Before surgery, administration of corticosteroids for 24–48 h may help to control peritumoral edema. Electrolyte imbalance, secondary to vomiting, should be carefully corrected. Medulloblastomas are usually richly vascularized tumors; thereafter adequate venous access (two to three peripheral venous catheters and a central venous line) is required. An arterial line for blood pressure monitoring is also mandatory.
The bladder is catheterized, and pulse oximetry, end-expiratory carbon dioxide, electrocardiography, blood pressure, body temperature are monitored.
For tumors that involve the brain stem, the use of motor, somatosensory, brain stem auditory evoked response, electromyographic monitoring of the lateral rectus and facial muscles, and direct electromyographic stimulation of relevant cranial nerves may be beneficial [17].
In our department, at the end of the procedure, it is customary to transfer the patient in ICU and keep him under controlled ventilation and sedo-analgesia until the next morning. We adopt this policy to avoid sudden modifications in arterial or venous pressure due to crying/coughing in the immediate postoperative hours and to allow a safer and easier postoperative MRI evaluation the following day. Mandatory prerequisite for this postoperative management is to achieve, by any means the surgeon prefers (ETV, EVD, VP shunt), preoperative control of hydrocephalus for tumors involving the fourth ventricle and the CSF pathways. In fact, the real major risk in posterior fossa surgery in children is acute postoperative hydrocephalus due to cerebellar swelling that typically occurs within the first 18–24 h after tumor removal. Postoperative swelling is less frequent in hemispheric lesions not involving the fourth ventricle. These lesions are usually approached through corticotomy and do not require vermian incision or significant cerebellar retraction. Postoperative swelling instead is very frequent in case that vermian incision, even partial, is required for approach or in cases with extensive parenchymal infiltration on the midline vermis that usually require more significant parenchymal retraction/manipulation even following telovelar approach. If swelling occurs, rapid obstruction to CSF pathways at the level of the outlets of the fourth ventricle may result, with consequent acute hydrocephalus with intracranial hypertension. If not resolved immediately with CSF drainage, this situation will result in downward and upward cerebellar herniation that may be rapidly fatal.
16.4 Operative Technique
16.4.1 Positioning
Despite some advantages of the sitting position, above all improved blood and CSF drainage from the operative field, in our institution, the prone position is preferred for all midline posterior fossa approaches. In fact there is a decreased risk (if not nil) of air embolism and the absence of postoperative pneumocephalus that is instead a constant significant finding following posterior fossa surgery in the sitting position. Moreover the surgeon works more comfortably, and there is less risk of tearing bridging veins between the cerebellum and tentorium than in the seated position [18]. In rare cases in which an EVD is required (as discussed above, we prefer to perform ETV in case of urgent treatment of hydrocephalus), we prefer to place it frontally prior to positioning rather than occipitally when positioning is completed.
In older children the head can be fixed in a rigid pin-type head holder, such as Mayfield® or Doro® systems. In Doro® system, up to four pins can be used to widely distribute the total force. In very young babies, the pins can be replaced by silicone pillows. A horizontal pillow placed on the horizontal arm of the Mayfield® or Doro® frame offers additional support to the forehead, increasing the safety of the system. In alternative, a well-padded horseshoe headrest can be used. In this case, great care must be paid to avoid compression of the ocular globes due to the horseshoe arms. If an intraoperative image system, such as intraoperative CT scan, is used, the positioning and fixation of the head need to be adapted with dedicated material. For the last 3 years, we have routinely used intraoperative CT scan: we prefer to fix the head in a CT compatible horseshoe headrest (Fig. 16.7).


Fig. 16.7
Prone position with the head fixed in a CT compatible horseshoe headrest for intraoperative CT scanning. (a: lateral view, b: frontal view, through the scanner)
Flexion of the neck with reverse Trendelenburg positioning of the torso is very important because it allows for visualization of the rostral part of the posterior fossa, i.e., tentorium, pineal region, and cerebral aqueduct. Venous drainage is improved; as like dissection of the occipitocervical musculature [6]. However, care should be taken to avoid over flexion: it may be dangerous in case of tonsillar herniation through the foramen magnum, and may prevent adequate venous drainage through neck veins.
The iliac crests are supported with bolster; the abdomen is left free to avoid venous engorgement; the shoulders should be supported with adequate padded pillows to slightly project beyond the operating table, so that flexion of the neck will not place the endotracheal tube close to the table. In obese children silk tape should be used to pull the shoulders caudally and place the occipitocervical musculature under tension. All pressure points should be padded.
16.4.2 Surgical Approach
For most medulloblastomas, a standard midline posterior fossa approach, described in more details in another chapter of the present book, can be used.
A midline incision from the inion to C5 is traced. The dissection is taken through the avascular midline. Unless imaging studies suggest tumor extending caudally beyond C1, one should avoid taking the paraspinous muscles off the laminae of C2: this contributes to postoperative pain and may increase the risk of postoperative cervical instability.
At our institution, a craniotomy, rather that craniectomy, is used for all posterior fossa approaches. Two paramedian burr holes are drilled just below the lateral sinuses, on either side of the midline. Following the first burr hole, if the lateral sinus is not visualized, further bone is removed cranially until visualization of the sinus, in order to exactly locate it and the torcula. The position of the second burr hole is modified accordingly. Another two more caudal and more lateral burr holes, on the cerebellar hemispheres, are drilled, in order to help dissecting the foramen magnum before craniotomy. The craniotome can then be used to turn as large a craniotomy as possible, joining the four burr holes and the foramen magnum. Usually it is necessary to further increase the bone exposure, with the help of rongeurs, especially at the level of the foramen magnum. If imaging studies reveal tumor below the level of the obex or tonsils caudally displaced, the posterior arch of C1 should also be removed, with the help of rongeurs.
Dura mater is opened in a Y shape, with the base along the lateral sinuses and midline durotomy extended caudally. Bleedings from the dura and occipital sinus should be controlled with dural clip or circumferential sutures with the technique. Every effort should be done to avoid mono or bipolar cautery on the dura. The dural flap should be retracted upward with sutures to expose as much cerebellum as possible. The retracted dura should be left retracted upward and laterally under tension provided by retracting sutures, protected with a wet patty between the dura and the bone and a second wet patty above the dura. If a “Y” incision is done, two additional retracting sutures should be placed in the two points joining the cervical dura incision with the cerebellar dura incision, retracting the dura laterally towards the paraspinal muscles and keeping the dura under tension. Two additional long wet patties should be placed vertically under the cervical dura and under the retracting sutures, allowing to keep the cervical dura wet and avoiding blood to enter the surgical field from the extradural space at the level of the occipital foramen. Frequent moistening of these patties should be ensured by the scrub nurse. Absolute, perfect hemostasis of the extradural space and a perfectly clean operative field should be obtained before starting microsurgical dissection.
16.4.3 Microsurgical Dissection
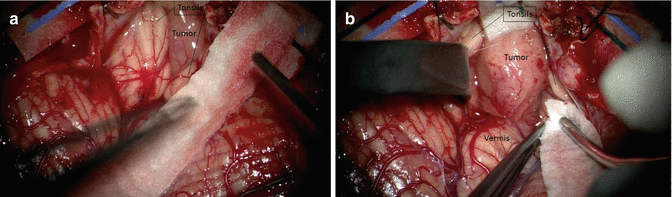
If the medulloblastoma (like in the vast majority) is located into the fourth ventricle, microsurgical dissection begins with opening of the cistern magna (Fig. 16.8). This may help in relaxing the brain, releasing CSF. Many surgeons sample the CSF from cistern magna to determine if there are malignant cells on cytology (Fig. 16.9) [19]. Usually the tumor grows underneath the vermis, progressively filling the fourth ventricle and underneath between the cerebellar tonsils: initial exposure may be facilitated by splitting the tonsils if the tumor has not already done so (Fig. 16.10). The initial goal of dissection is to find and protect the floor of the fourth ventricle. If the tumor is not adherent at the level of the obex, microsurgical dissection of the arachnoid about the vermian peg will allow for elevation of the inferior pole of the tumor and visualization of the distal floor (Fig. 16.11).


Fig. 16.8
Microsurgical dissection of the cisterna magna