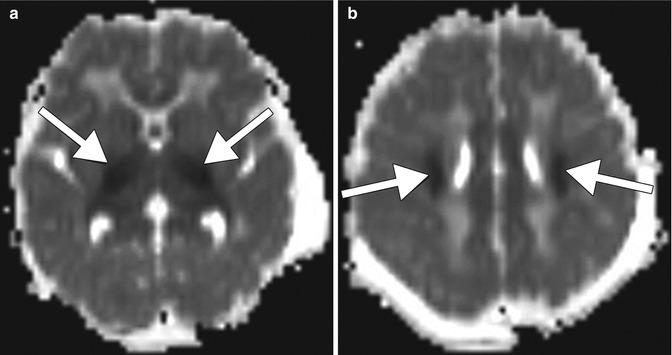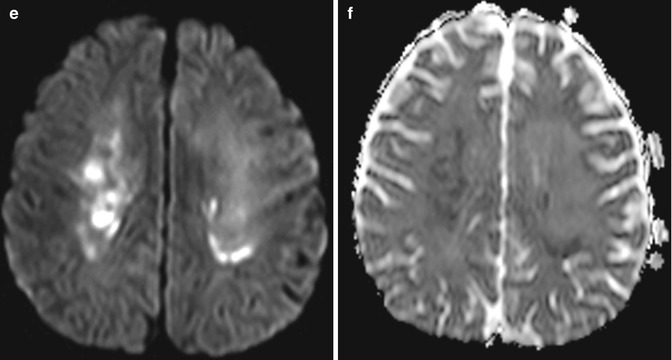
Fig. 19.1
Methotrexate leukoencephalopathy. Axial FLAIR (a), axial DWI (b), and ADC maps (c) show patchy areas of FLAIR hyperintensity in the bilateral white matter of the centrum semiovale with corresponding marked restricted diffusion (arrows). Follow up axial FLAIR (d), axial DWI (e), and ADC maps (f) obtained one week later show more pronounced FLAIR signal abnormality, but the restricted diffusion has largely subsided. There was no associated abnormal enhancement (not shown)
A more unusual neurotoxic complication of methotrexate is focal brain necrosis after intraparenchymal infusion of methotrexate, which may be caused by catheter disconnection of the Ommaya device. This can result an area of cytotoxic edema adjacent to the catheter track, which in turn is surrounded by more extensive vasogenic edema (Fig. 19.2). Besides halting methotrexate administration, treatment options for methotrexate neurotoxicity include aminophylline, an adenosine antagonist, and dextromethorphan, an antagonist of the NMDA receptor.
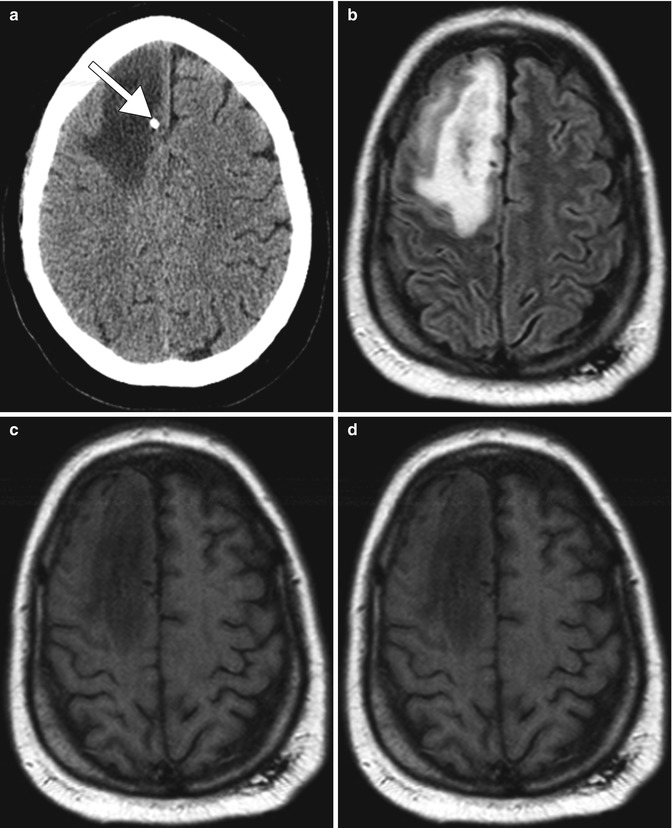
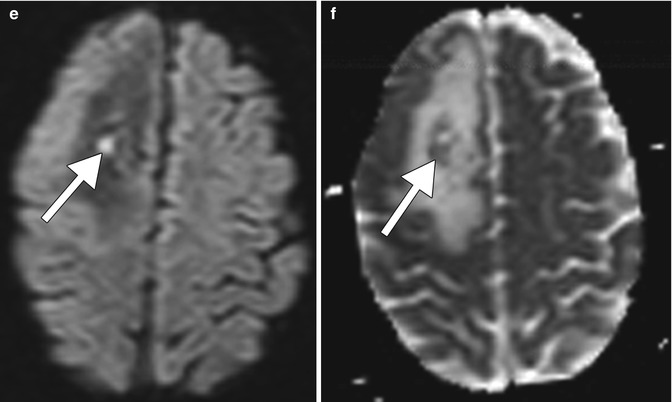


Fig. 19.2
Focal brain necrosis. Axial CT image (a) shows extensive confluent hypoattenuation in the right frontal lobe white matter surrounding the Ommaya catheter (arrow). Axial FLAIR MRI (b), DWI (c), and ADC map (d) show extensive vasogenic edema surrounding a more focal area of restricted diffusion surrounding the catheter (arrows)
Adhesive arachnoiditis that develops after administration of intrathecal methotrexate can cause urinary retention or incontinence in severe cases. The symptoms can begin within hours of administration and sometime resolve. On MRI, adhesive arachnoiditis can appear as clumping of the nerve roots to one another or to the thecal sac (Fig. 19.3). There may or may not be associated enhancement.
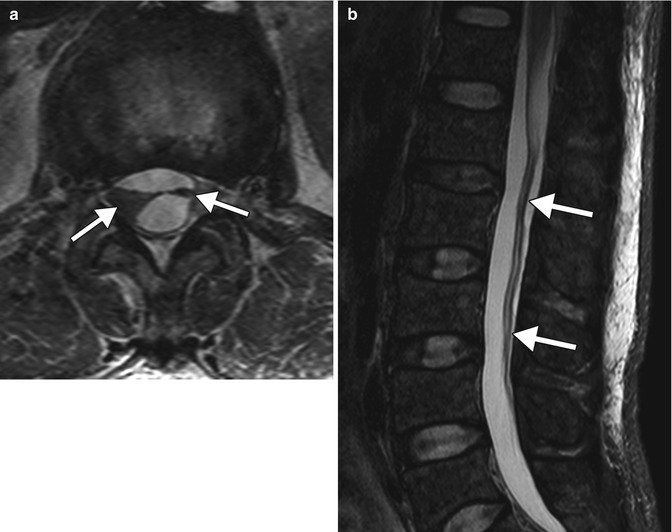

Fig. 19.3
Intrathecal methotrexate-induced adhesive arachnoiditis. Axial (a) and sagittal (b) T2-weighted MR images show extensive clumping of the cauda equine nerve roots (arrows) (Courtesy of Gregory Christoforidis)
Compared to encephalopathy, methotrexate-induced myelopathy is rare. Risk factors for development of methotrexate myelopathy include cumulative intrathecal methotrexate doses, systemic methotrexate, concurrent radiotherapy, and central nervous system involvement of primary disease. On MRI, the myelopathy appears as long-segment T2 hyperintensity within the dorsal columns (Fig. 19.4). The dorsal column signal changes followed by lateral column changes on serial MRI and the gradual caudal-to-rostral extension of lesions are analogous to subacute combined degeneration due to vitamin B12–folate deficiencies. However, a normal spine MRI does not exclude methotrexate myelopathy. Methotrexate myelopathy often has a poor prognosis and can occur in conjunction with arachnoiditis. Treatment with folate metabolites may improve outcome.
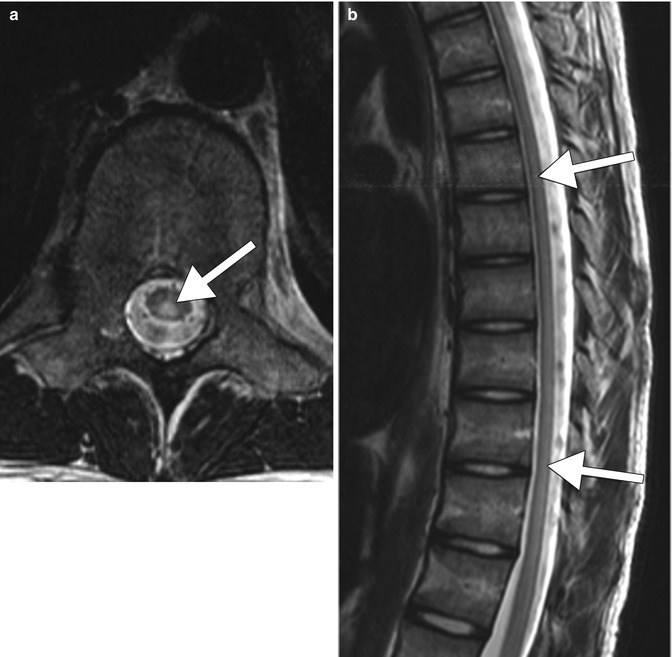

Fig. 19.4
Methotrexate myelopathy. Axial (a) and sagittal (b) T2-weighted MR images show long-segment hyperintensity affecting the dorsal columns of the thoracic spinal cord (arrows)
Mineralizing microangiopathy consists of dystrophic calcifications in the basal ganglia, subcortical white matter, and dentate nuclei. These are a relatively common finding at cranial CT in children previously treated with radiation therapy and intrathecal methotrexate. On MRI, high signal on T1-weighted images is observed in the putamen and decreased signal is on T2-weighted images (Fig. 19.5). These imaging findings appear 2 or more years after treatment. The presence of mineralizing microangiopathy on imaging does not clearly correlate with clinical symptoms.
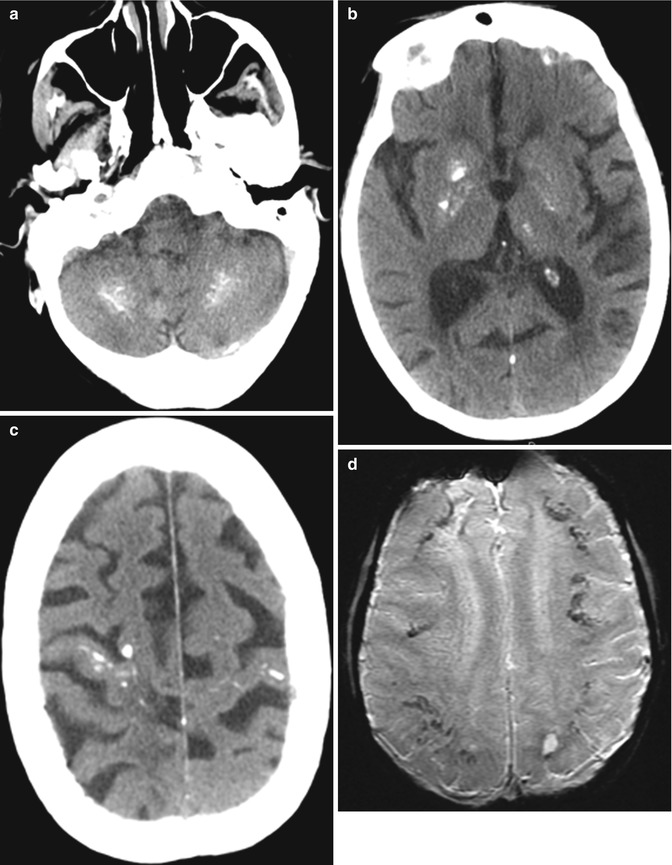
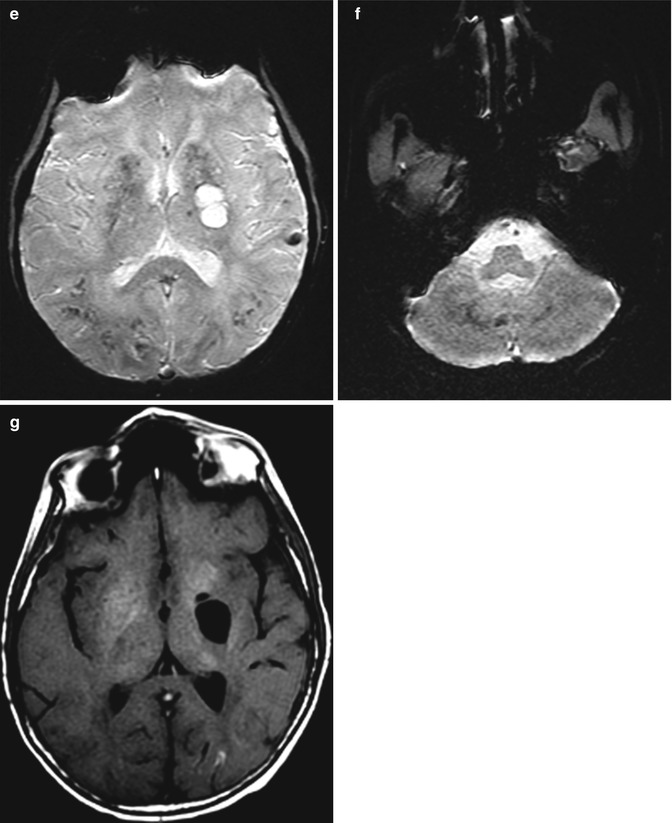


Fig. 19.5
Mineralizing microangiopathy. Axial CT images (a–c) show calcifications in the bilateral perirolandic subcortical white matter, basal ganglia, and dentate nuclei. The T2* GRE MR images (d–f) show corresponding low signal in the affected areas. In addition, the axial T1-weighted image (g) shows high signal within the basal ganglia
19.4 Differential Diagnosis
Encephalopathy: Methotrexate-induced encephalopathy resembles delayed leukoencephalopathy with a stroke-like presentation observed with a variety of chemotherapeutic agents. Other differential considerations include hypoxic ischemic encephalopathy (Fig. 19.6), PML, PRES, ADEM, and hereditary demyelinating neuropathies, such as Charcot–Marie–Tooth disease (Fig. 19.7). Restricted diffusion in the splenium of the corpus callosum can result from the use of other medications, such as metronidazole toxicity (refer to Chap. 25).