Chapter 42 Microsurgical Approaches to the Ventricular System
• Intraventricular lesions often present with nonspecific symptoms and clinical signs and can affect patients of all ages. They may grow quite large before the diagnosis is secured. Imaging studies, in concert with a detailed knowledge of the surrounding microsurgical anatomy and neural function, permit the surgeon to determine the most effective and safest approach to resection of the lesion.
• The key principles in the microsurgical resection is to approach these deep-seated lesions by the most direct route that provides the least disruption to eloquent cortex and neural function. In addition, the surgical goal includes developing the most favorable trajectory for visualization of the entire lesion, the best proximal control of arterial feeders, least disruption to the venous drainage, and minimal contamination of the cerebrospinal fluid (CSF).
• Transcortical or interhemispheric transcallosal approaches adequately access lesions throughout the entire ventricular system. Transcortical approaches are ideal in patients with hydrocephalus, and interhemispheric approaches work regardless of the ventricle size. Both approaches permit the full range of microsurgical options to enter the third ventricle through the choroidal fissure or between the fornices.
• The midline telovelar approach is a safe and effective approach to resect fourth ventricle lesions extending from the aqueduct of Sylvius to the obex, without having to split the vermis.
• Complete ventricular communication after tumor resection is an important goal. Cystic septations should be removed so that CSF spaces communicate both anatomically and physiologically. Microscopic or endoscopic inspection at the end of the procedure confirms the absence of a blood clot, residual tumor, or septations and ensures that transventricular communication has been achieved.
• Microsurgical approaches are to be tailored to the lesion location, pathological entity, and surgeon’s experience. Each approach has distinct technical advantages with associated functional risks. Endoscopic approaches can be used to achieve resection of select intraventricular lesions, most commonly colloid cysts of the third ventricle. Future advances in endoscopic instrumentation, preoperative functional imaging, and fiber tract identification will influence surgical strategies in the coming years.
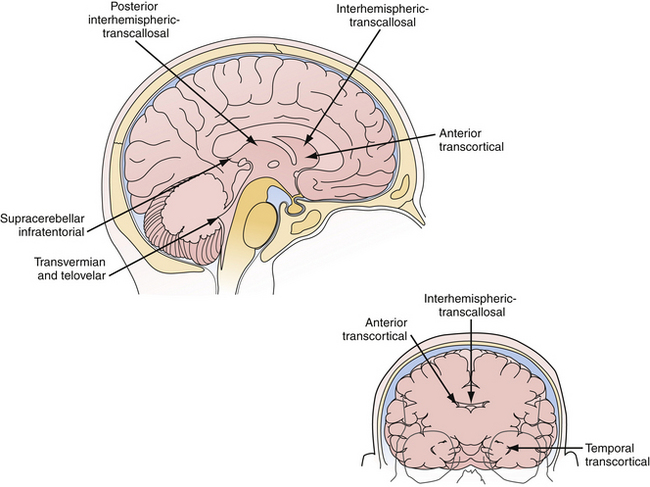
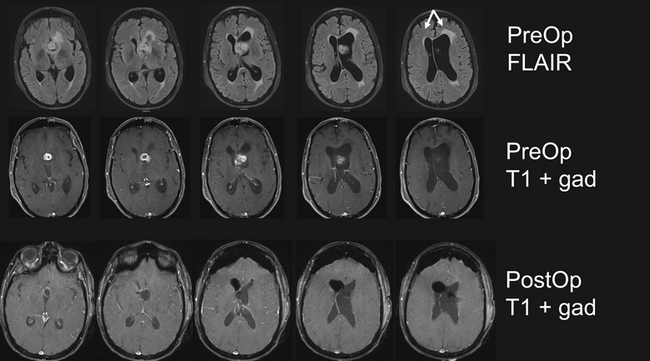
Microsurgery for ventricular tumors is both challenging for the surgeon and potentially curative for the patient. Accounting for a small percentage of all cerebral lesions,1 tumors of the ventricles present a unique clinical and elegant microsurgical experience for neurosurgeons. Intraventricular tumors can grow to a very large size, eluding diagnosis until they cause rapid decompensation from hydrocephalus or increased intracranial pressure. Surgical approaches for resection of lateral and third ventricle tumors always require the neurosurgeon to enter through the brain, either the cerebral cortex or corpus callosum. Complete resection of these lesions is often possible through either the transcortical or interhemispheric transcallosal routes. Surgical approaches to the fourth ventricle are different in that splitting of a fissure through a telovelar approach may provide access of the fourth ventricle from the aqueduct of Sylvius to the obex without sacrifice of any cerebellum (Fig. 42.1).
The age of presentation of intraventricular tumors spans from infancy to the elderly.2 Preoperative symptoms are often nonspecific; including headache (35%), weakness (25%), sensory loss (25%), nausea and vomiting (22%), dementia (18%), and visual loss (18%).2–7 Pathological entities range from slow-growing indolent lesions to aggressive malignancies. Tumors arising within the third and lateral ventricles are frequently (but not always) slow-growing and benign,4,5 whereas lesions arising from the fourth ventricle may be rapidly progressive due to cerebrospinal fluid (CSF) obstruction and brainstem signs and include both benign and malignant pathological types.
Despite these challenges, surgery for intraventricular tumors can be rewarding for the patient. When the symptoms are primarily related to the hydrocephalus resulting from the obstructive nature of these tumors, clinical improvement may be immediate. Gross total resections of low-grade lesions may be curative. Finally, the refinements in technique and equipment have improved outcomes substantially since Dandy’s first description of surgery for intraventricular tumors in 1922.8
Microsurgical Anatomy
Mastering the relevant ventricular anatomy is essential in performing microsurgical approaches to the lateral ventricles. A more detailed review of the anatomy can be found in Professor Albert L. Rhoton’s outstanding monograph on the subject, which was produced after years of study in his laboratory and operating room.9
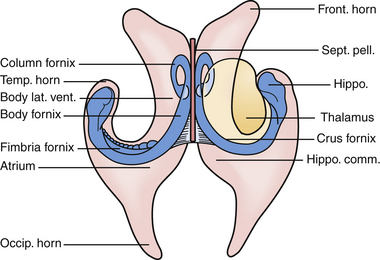
The lateral ventricles are paired C-shaped structures that are anchored by each thalamus in the middle of the brain. Each ventricle is divided into five sections. Starting anterior and superior and moving backward they are the (a) anterior horn, (b) body, (c) temporal horn, (d) atrium, and (e) occipital horn (Fig. 42.2). Each of these five sections has a floor, roof, medial wall, and lateral wall and these sections contain structures that are critical to the surgeon for navigation in the ventricles. Specifically, they are structures that either tolerate manipulation to facilitate visual access or, conversely, should not be manipulated. These relationships are important when considering either the transcortical or the transcallosal approach.
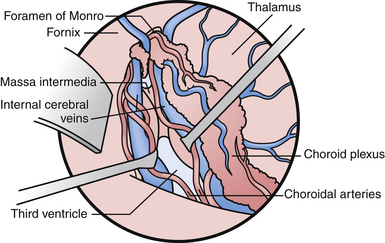
If one visualizes the ventricles in a coronal plane, the anatomical relationships are as follows. At the level of the frontal horn of the lateral ventricle, the lateral wall is composed of the oval caudate nucleus, and the medial wall is the diaphanous septum pellucidum that separates the two frontal horns. The roof is made up of the roof of the genu of the corpus callosum, and the floor is composed of the rostrum of the corpus callosum, which wraps around and tucks under the ventricles until it reaches the anterior commissural fibers. At the level of the body of the ventricle, some of the same relationships exist. The corpus callosum makes up the roof, the caudate nucleus the lateral wall, and the medial wall is again separated by the septum pellucidum. Moving posteriorly within the lateral ventricles, the floor is composed of the thalamus. In the midline of the floor lie the columns of the fornix. In the floor of the body of the lateral ventricle, sitting between the fornix and the thalamus, is the choroid plexus, which attaches to the surgically crucial structure called the choroidal fissure. Each column of the fornix also forms the curved superior and anterior borders of the foramen of Monro. Moving to the temporal horn, each column of the fornix starts as the fimbria of the hippocampi and starts in the medial wall of the temporal horn. After giving off commissural fibers, these nerve bundles continue in the inferior medial wall of the body as the fornix.10 The roof of the temporal horn is the tapetum of the corpus callosum, and the floor comprises the folded gyri of the hippocampus. Moving within the ventricle to its posterior limits we find the atrium and the occipital horn. The atrium and occipital horn form a triangular CSF cavity in the occipital lobe. Again, the tapetum of the corpus callosum covers the lateral wall and roof of the atrium. The forceps major, which is a fiber bundle that connects the two occipital lobes through the splenium of the corpus callosum, runs in the superior part of the atrium. The floor is composed of the collateral trigone and the medial wall is the calcar avis of the calcarine cortex.7
The primary arteries within the ventricles are the anterior and posterior choroidal arteries,7,10 branches of which provide the vascular supply to tumors in this region. Understanding the course of the arteries helps the surgeon choose an approach for each lesion and thus permits early proximal control, when possible, of the feeding vessels.
The anterior choroidal artery arises from the internal carotid artery, just a millimeter or so distal to the posterior communicating artery. It exits the anterior incisural space and enters the lateral ventricle through the choroidal fissure, heading posteriorly to lie near the lateral posterior choroidal artery.7,10 The anterior choroidal artery generally supplies the choroid plexus in the temporal horn and atrium. Because the choroidal arteries pass through the choroidal fissure, opening this fissure early also facilitates proximal control of the feeding vessels.
The posterior choroidal arteries are grouped into lateral and medial divisions. The lateral posterior choroidal artery is composed of one to six branches, which arise in the ambient and quadrigeminal cisterns, typically from the posterior cerebral aratery (PCA). These branches then pierce the ventricle and pass around the pulvinar and enter through the choroidal fissure at the level of the fimbria/body of the fornix to supply the choroid plexus in the posterior temporal horn, atrium, and body of the ventricles.7 The medial posterior choroidal arteries arise as one to three branches from the PCA in the interpeduncular and crural cisterns. These arteries circumnavigate the midbrain and move to the pineal gland to enter the roof of the third ventricle and reside in the tela choroidea called the velum interpositum, adjacent to the internal cerebral veins. The medial posterior choroidal artery supplies the choroid plexus in the roof of the third ventricle and sometimes the choroid plexus of the lateral ventricle.10
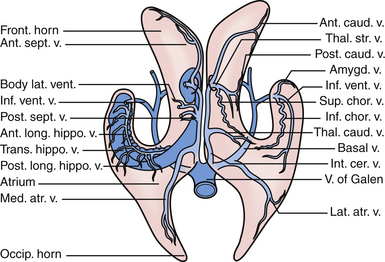
The veins are useful as landmarks to help navigate in the body of the ventricle, especially in cases in which hydrocephalus is present (Fig. 42.3). There are many important veins composing the lateral and medial groups, but perhaps the best known for surgical and angiographic orientation is the thalamostriate vein, which helps orient the surgeon toward the foramen of Monro. The thalamostriate vein traverses the lateral wall of the body of the ventricle adjacent to the choroidal fissue between the caudate and thalamus. It then forms the venous angle with an acute posterior turn into the foramen of Monro to empty into the internal cerebral veins traversing the velum interpositum. The veins in the temporal horn drain into the basal vein of Rosenthal (basal vein) as it passes through the ambient cistern on each side. Veins from the atrium and occipital horn drain into the basal internal cerebral veins as well, and finally into the vein of Galen which empties into the straight sinus and torcular herophili.7
Pathological Entities
The differential diagnosis of tumors in the lateral ventricle depends on several factors: the age of the patient, location of the tumor, and specific radiological characteristics as described by computed tomography (CT), magnetic resonance imaging (MRI), and cerebral angiography.11,12 Tumors found in the lateral ventricle of children younger than 5 years of age are often choroid plexus tumors, whereas in older children they tend to be astrocytoma or ependymoma.11 Choroid plexus tumors are rare lesions with a prevalence of 0.3 per million. They comprise only about 1% of all brain tumors and occur in children with a median age of 3.5 years at the time of presentation.13,14 Choroid plexus tumors are often quite vascular and demonstrate a tumor blush on CT, MRI, and cerebral angiography. On MRI, they may possess heterogeneous signal characteristics caused by necrosis and calcification and are iso- to hypodense on T1-weighted images relative to the white matter.
The most common hypo- or isodense, nonenhancing tumor in the body or foramen of Monro is a subependymoma. In children with tuberous sclerosis (TS), these lesions are often a subependymal giant cell astrocytoma (SEGA). Roughly 6% of patients with TS develop subependymal giant cell tumors.15 In this location, they can reach a large size before being diagnosed, sometimes by unilateral ventricular obstruction.16–18 Astrocytomas can be found in any part of the ventricle as they are surrounded by white matter tracts. Astrocytoma will most frequently arise from the thalamus, where they can infiltrate. Ependymoma, when supratentorial, can be intraventricular as well as intraparenchymal.11
In adults older than 30 years of age, tumors in the atrium and trigone are most commonly meningiomas. Intraventricular meningiomas are well-circumscribed, homogeneously enhancing lesions.19 Meningiomas tend to be isodense to brain on T1-weighted images and brightly enhance with gadolinium administration. Intraventricular masses that engulf the choroid glomus, are calcified, or are demonstrated to have a choroidal artery supply on angiography are usually benign meningiomas.20,21
Outside the trigone, tumors in older patients are often either a primary or metastatic malignancy.11
Primary or metastatic malignancies present late in life with attachment to the ventricular walls and parenchymal invasion.11 Malignant intraventricular metastases include renal cell carcinoma, pulmonary adenocarcinoma, gastrointestinal carcinoma, transitional cell carcinoma, and adrenocortical carcinoma.
Central neurocytomas, which occur mostly in adults in the second to fourth decades of life, appear to attach or grow from the septum pellucidum and possess a characteristic imaging appearance.22 These lesions may be solid with cystic components and flow voids on MRI, and may contain calcifications visualized on CT.23 These benign World Health Organization (WHO) grade II lesions derive their name from the original description of a well-differentiated neural lesion that was adherent to the septum and ventricle wall.24 These tumors can be quite large on presentation. Although the natural history and long-term prognosis of these lesions are not completely understood, excision appears to be curative in those patients in whom a gross total resection is achieved. Significant blood loss and a “sticky” connection to the thalamus and third ventricle structures may complicate the attempts at a radical resection.
Cystic lesions include colloid cysts of the third ventricle and infectious lesions such as neurocysticercosis, nocardiosis, and cryptococci. Colloid cysts are covered in another chapter but at our institution they are routinely approached with a single port endoscopic resection. Other non-neoplastic processes such as cysts, sarcoidosis, xanthogranulomas (which appear to be dense on CT scans with flecks of calcification), arteriovenous malformations, and cavernous hemangiomas are also found in the lateral ventricle with geographic variation.25 In some endemic regions of the world, a new seizure or hydrocephalus associated with cystic intraventricular lesion often indicates neurocysticercosis. In our institution, the history, MRI, and serum or CSF markers may secure the diagnosis of neurocysticercosis, and these patients then receive medical therapy. Endoscopic exploration and resection are used to remove obstructive ventricular neurocysticercosis lesions when indicated by symptoms or examination.
Preoperative Planning and General Surgical Considerations
A CT scan is often the first radiological study patients undergo simply because of diagnostic ease in the surveillance workup. The presence of calcifications on the CT image may further narrow the differential diagnosis.26 Calcification may be seen in central neurocytoma, subependymal giant cell tumor, meningioma, and ependymoma.
Subsequent MRI, with and without gadolinium, reveals the precise location and extent of the lesion within the ventricle and, thus, helps guide the surgical approach. At a minimum this should include sagittal, coronal, and axial reconstructions of T1-weighted sequences (with and without gadolinium), T2-weighted images, and fluid-attenuated inversion recovery (FLAIR). These sequences are sufficient to refine the differential diagnosis and begin basic surgical planning. The role of new specialized MRI sequencing is rapidly evolving. Diffusion tensor imaging (DTI) permits reconstruction of large fiber tracts, which may contribute to surgical planning by guiding the surgical trajectory through the least disruptive pathway.27 Functional MRI (fMRI) has a role in cases in which the approach encroaches on motor or language cortex. Some authors advocate magnetic resonance spectroscopy (MRS) to characterize the chemical composition of the lesion in malignant lesions that may require a biopsy.28 However, we have found the DTI and fMRI to be more helpful in surgical planning. It is our practice to also obtain magnetic resonance angiography (MRA)and venography (MRV). These images serve several purposes. First, they permit visualization of the blood supply to the lesion. Second, MRV through the vertex allows the surgeon to tailor the placement of the craniotomy based on draining cortical veins. Finally, MRV is helpful when considering choroidal dissection to gain access to the third ventricle.29
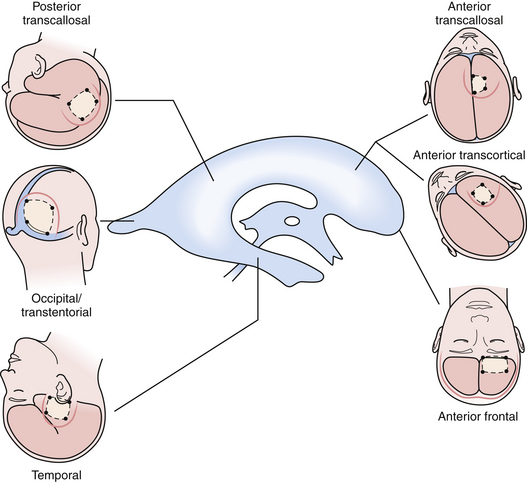
The beautiful anatomy of the lateral ventricle facilitates a wide variety of surgical approaches. The location and size of the lesion, hemispheric dominance, preoperative deficits, associated hydrocephalus, vascularity of the lesion, and experience of the surgeon all contribute to the ultimate selection of surgical approach. There is simply no class I or II data that dictates one approach is superior to another. The optimal approach facilitates the primary goal of surgery, which is gross total resection of the lesion with minimal trauma to surrounding structures and neural function. At our institution, most lateral ventricle lesions are approached through a transcortical route, a transcallosal route, or in rare cases a combination of the two. As detailed in the following sections, these routes provide excellent access to lesions within the third and lateral ventricles (Fig. 42.4).
Craniotomy Flap and Cortical Incisions
To enter the ventricle directly, we employ a simple technique of placing a ventricular catheter by frameless stereotactic guidance, or through a freehand pass to enter the ventricle. Once the trajectory to the ventricle is determined, a small cortical incision is made and followed down to the catheter in the ventricle. The ependyma is opened with nonstick or irrigating bipolar electrocautery, and the ventricle is entered. A cortical aperture is made using flat microinstruments or microsuction to develop a subcortical path to the lesion. Very little of the subcortical white matter is removed, and flat malleable brain retractors or a microsurgical speculum is placed to increase visibility. The goal is not necessarily to make the smallest cortical incision but to make the most appropriate one. Transcortical surgery has the advantages of wide avenues and a maneuverable microsurgical field.30
There are limitations to the transcortical approach. This approach may not provide optimal visualization of the contralateral ventricle due to the linear trajectory, which may obscure lesions sitting in a location out of the line of sight of the microscope. The transcortical approach may also predispose the patient to postoperative seizures. The incidence of postoperative seizures ranges in the literature from 5% to 70%,2,3,31–34 which is higher than in transcallosal approaches when no cortisectomy is performed (0-10%). However, our experience with seizure activity after the transcortical approach parallels that of the transcallosal approach. Specifically, the postoperative seizure rate with very small corticectomy (<15 mm) and prophylactic anticonvulsant treatment for 1 week has successfully lowered the immediate postoperative seizure incidence to the 5% to 7% range.
Anterior Transcortical Approaches
Tumors in the frontal region are primarily astrocytoma, subependymal giant cell astrocytoma, ependymoma, and central neurocytoma. Tumors of the frontal horn can become very large and cause obstruction of the foramen of Monro with ventricular dilation. The transcortical middle frontal gyrus approach is an excellent route for the excision of tumors in the ipsilateral anterior horn of the lateral ventricle, the anterior body of the lateral ventricle, and the anterior third ventricle. Tumors that extend inferiorly from the lateral ventricle into the third ventricle and require an interforniceal or subchoroidal exposure for removal, can be approached using either a transcortical or a transcallosal route.10 In patients with small ventricles, tumor in both lateral ventricles, or tumor in the body of the lateral ventricle, the transcallosal approach is employed.
The patient is placed in the supine position with the head elevated 10 to 30 degrees. A free 3 × 4-cm bone flap is placed over the central portion of the middle frontal gyrus. The flap is based on the coronal suture, with the medial border off the midline and the anterior border at least 2 cm anterior to the coronal suture and the posterior border about 2 cm behind. The dura is opened in a cruciate or box-shaped fashion and a ventricular catheter is placed into the frontal horn. This is followed by microsurgical cleavage of the white matter until the ependymal lining is broached. A small ⅜-inch retractor or speculum retractor is used. After the anatomy of the lateral ventricle is visualized through the microscope, regardless of the approach, the anatomy is similar. Once inside the ventricle, surgical landmarks provide orientation. The most important landmarks are the foramen of Monro, the thalamostriate vein, the choroid plexus, and the fornix. Cotton paddies are placed over the foramen and around the lesion to minimize the circulation of blood products and debris during tumor resection.
Lesions within the third ventricle may be accessed from the transcortical approach. If the foramen is enlarged from hydrocephalus or the lesion itself, no additional dissection is necessary. If not, additional access to the third ventricle is made possible by a transchoroidal or suprachoroidal, trans–velum interpositum dissection.35 To achieve this exposure, the choroidal fissure is opened between the fornix and the thalamus.36 The suprachoroidal or transchoroidal approach divides the taenia of the fornix between the fornix and choroid plexus. The subchoroidal approach divides the taenia thalami leaf of the tela choroidea between the choroid plexus and the thalamus. The latter approach is more commonly associated with direct injury to the thalamostriate vein and thalamus. Rhoton’s elegant lifetime work has been captured in his collected works in Neurosurgery and is arguably the most complete work on the subject.9,37 In his ventricular surgery experience, he has demonstrated that the transchoroidal or suprachoroidal approach, opening the fissure through the taenia fornix, is both safe and effective.18 Although we have safely opened the fissure through the “subchoroidal” approach on the taenia thalami side, the risk of thalamic damage is quite real and can be devastating. In contrast, opening on the taenia fornix side in which a unilateral forniceal injury occurs often results in no permanent memory loss (Fig. 42.6). Regardless, these aforementioned considerations are important but must be applied to the specific anatomy at the time of the intraventricular dissection. Complications from injury to the surrounding structures result in hemiparesis, mutism, amnestic syndromes, and confusion.38 In general, these risks are minimized by a suprachoroidal approach. However, maintaining the integrity of both fornices is important for preservation of memory function. The fornix, which has been reported to carry as many as five times the number of axons as the optic tract,39 links the hippocampal formation (including the hippocampus proper, dentate gyrus, parahippocampal gyrus, and subiculum) with the septal nuclei, mammillary bodies, hypothalamus, and thalamic nuclei. Visualization of the fornix may be obscured by lesions based on the septum pellucidum, such as central neurocytomas. Patient dissection and meticulous technique here will be rewarded with a favorable postoperative outcome.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree