CHAPTER 163 Microvascular Decompression for Trigeminal Neuralgia
History
TN has been known since ancient times. Two thousand years ago, Aretaeus of Cappadocia referred to headache with “spasm and distortion of countenance,” and Persian scholar Avicenna described a similar syndrome of facial pain in the 10th century.1 In the 17th century, Fehr and Schmidt described the syndrome in a eulogy.2 John Locke described facial pain of the Countess of Northumberland, wife of the English Ambassador to France, as a “fit of such violent and exquisite torment that it forced her to … cries and shrieks … which extended itself all over the right side of her face and mouth.”1 In 1756, Nicolas Andre coined the term tic douloureux, and described it as “a cruel and obscure illness, which causes … in the face some violent motions, some hideous grimaces which are an insurmountable obstacle to the reception of food, [and] which put off sleep.”3 The first comprehensive clinical description occurred in 1773 when John Fothergill wrote to the medical society of London about 14 patients with TN, and his descriptions of triggerable lancinating pain are still considered accurate.4
Early surgical treatments for TN involved intentional lesioning of the trigeminal nerve. In 1934, Walter Dandy described the retrosigmoid approach to the trigeminal nerve and noted frequent vascular contact in patients with TN. He wrote, “In many instances the nerve is grooved or bent in an angle by the artery. This I believe is the cause of tic douloureux.”5 Despite his insight, Dandy did not attempt to decompress the nerve, believing the pathologic process to be irreversible. Microvascular decompression (MVD) was first performed by W. James Gardner in 1959, who described mobilizing a vessel from the trigeminal nerve and placing a piece of Gelfoam between them without any intentional damage to the nerve itself.6 The procedure was subsequently refined and popularized by Peter Jannetta, who, with the aid of the operating microscope, performed thousands of MVD operations and demonstrated that long-term relief of pain is possible in most of appropriately selected patients.7–9
Pathophysiology
The etiology of TN is believed to be related to abnormal conduction within the trigeminal nerve, possibly owing to changes in myelin induced by pulsatile mechanical trauma from an adjacent vessel. At the point just before it enters the brainstem, there is a short segment where nerve axons are still ensheathed in central myelin (produced by oligodendrocytes), but after a few millimeters, there is a transition to peripheral myelin (produced by Schwann cells). The region of this transition is called the Obersteiner-Redlich zone. It is thought that the area of the nerve containing the central form of myelin is especially susceptible to pathologic changes from vascular contact that result in demyelination and altered conduction. Pathologic studies from patients with TN have demonstrated severe damage to myelin as well as axon loss within the nerve adjacent to the site of compression.10 The resulting conduction abnormality may lead to nerve hyperactivity owing to ectopic impulse discharge, spontaneous and triggered afterdischarge, and cross-excitation among neighboring afferent fibers (ephaptic transmission).10,11
Separation of the nerve from the offending vessel appears to immediately reverse many of the physiologic changes. In Leandri and colleagues’12 study of 10 patients undergoing MVD who underwent nerve root and scalp electrode recordings, 7 showed signs of immediate improvement in neurophysiologic parameters after decompression. Others authors have reported improvement in sensory thresholds for touch, pinprick, and temperature sensations after MVD13 as well as resolution of asymmetric jaw motion.14,15 These findings suggest that the changes associated with neurovascular compression are likely to be reversible if the nerve is decompressed, at least in the early stages.
Radiographic and anatomic studies have demonstrated that vascular contact with the trigeminal nerve is common even in asymptomatic individuals but tends to be more severe and more proximal on the nerve ipsilateral to TN symptoms.16 Patients with TN are more likely to have contralateral arterial compression than asymptomatic people even though bilateral TN is distinctly rare.16,17 Symptomatic and asymptomatic arterial compression of the trigeminal nerve increases with age because of elongation of cisternal arteries, which explains why it is primarily a disease of older adults.16
Alternative Treatments
TN symptoms often improve with medications that exert a stabilizing effect on neural conduction such as antiepileptics. Medications that have been successfully used include carbamazepine, phenytoin, valproate, gabapentin, pregabalin, baclofen, and clonazepam. Most patients obtain good pain control initially,18,19 but the effect tends to diminish over time, and after 10 years about half will no longer respond.20 Because the clinical and pathologic changes associated with TN may be progressive over time, initial failure of pharmacologic therapy may represent an indication to proceed with more aggressive treatment.21 Nevertheless, medical therapy is recommended as a first-line treatment for patients with TN because some patients will require no further treatment.
MVD differs from the other treatments in that the primary cause of TN is treated so that long-term pain relief is possible. Also, because there is no intentional damage to the nerve, facial dysesthesia and numbness are rare. Although it is the most invasive and expensive treatment, MVD is associated with the lowest rate of pain recurrence and the highest rate of patient satisfaction among all surgical treatments for TN.22 MVD can also be safely performed after a lesioning procedure and appears to be no less effective so long as there is no evidence of trigeminal neuropathy.23
Patient Selection and Classification of Facial Pain
MVD is ideal for young healthy patients with TN because no other treatment offers a significant likelihood of long-term pain relief.24 However, advanced age is not by itself a contraindication because there is no difference in complication rate or outcome in elderly patients.25–27 The operation is in fact technically easier in older patients because cerebellar atrophy leads to less need for retraction and less risk for cerebellar swelling. If life expectancy is very short or general anesthesia cannot be tolerated, a less invasive destructive procedure may be more appropriate.28,29
A careful history is essential during preoperative evaluation. Patients generally report intense stabbing or electric shock–like sensation, although there may be an overlying constant pain that may be more severe than the stabbing pain. Any distribution within the trigeminal nerve innervation territory may be observed. V2 and V3 branches are more common,7 especially radiating out from near the mouth. V1 symptoms are sometimes associated with decreased corneal sensation. The pain is often worse during the day and may be positional with relief when supine, with the affected side up, or during sleep. Trigger points are present in most patients and are activated by light cutaneous stimuli such as wind, eating, talking, and shaving. Often, the triggers lead to guarding of the face and refusal to be touched, wash, apply makeup, shave, or brush the teeth because of fear of an attack. Pain-free intervals lasting weeks to months are common at first but become rare as the syndrome progresses. Initial onset of pain is frequently quite memorable. Many patients undergo dental procedures without relief before a diagnosis is made. If the patient is given antiepileptic medication, pain usually improves dramatically. Physical examination is usually normal, although about one third of patients have some degree of sensory loss in the affected area.
When evaluating a patient for surgery, it is helpful to classify facial pain according to the classification scheme reported by Burchiel.30 Patients with TN type 1 have predominantly shock-like pain, whereas patients with TN type 2 report that at least 50% is constant pain, although there still may be a component of lancinating pain. Pain relief after MVD is more strongly correlated with the lancinating pain component than with any other symptom, so although most patients with either type will have long-term pain control, patients with TN type 1 are more likely to do well than those with TN type 2 (Miller and coworkers, unpublished data). Facial pain diagnoses other than TN are unlikely to improve after MVD. TN with a history of multiple sclerosis (MS) is called symptomatic trigeminal neuralgia (STN). MS is present in 1% to 3% patients with TN, and 2% to 4% of patients with MS have TN, probably due to intrinsic demyelination within the nerve or increased sensitivity to vascular trauma.31,32 Although MS patients sometimes improve after MVD, the recurrence rate is higher, and long-term elimination of pain is rare,33,34 so destructive procedures may be more appropriate for these patients.35 Sensory loss with burning pain is a sign of trigeminal neuropathic pain (TNP); if it occurs after a previous destructive procedure, this is called trigeminal deafferentation pain (TDP). Allodynia and dysesthesia with a history of herpes zoster suggest postherpetic neuralgia (PHN). MVD is not a good option for any of these patients. Atypical facial pain (AFP), which refers to pain of psychological onset, requires neuropsychological testing for the diagnosis and is unlikely to improve after MVD.
Preoperative Imaging
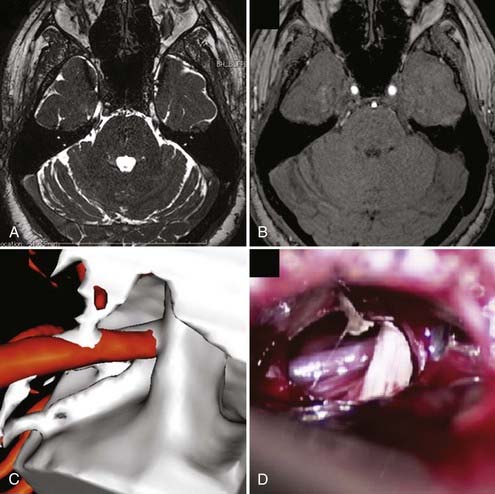
All patients should undergo magnetic resonance imaging (MRI) before MVD to verify that there is no intracranial mass lesion that may be responsible for the pain, such as a tumor, cyst, aneurysm, or arteriovenous malformation. MRI also can identify evidence of demyelinating disease or inflammatory changes and may reveal anatomic characteristics that would make MVD technically problematic, such as an ectopic basilar artery or bony abnormalities. High-resolution MRI using steady-state precession images can be used to identify neurovascular compression preoperatively with a high degree of accuracy, and fusion with magnetic resonance angiography followed by three-dimensional reconstruction can be used to simulate the intraoperative view for surgical planning (Fig. 163-1).36
Operative Technique
A cranial fixation device such as the three-pin Mayfield head holder is applied before positioning. There are several options for patient positioning for MVD. The simplest option is to place the patient in a flat supine position with the head rotated and flexed to the opposite side. Ideally, no shoulder roll should be used so that the ipsilateral shoulder does not obscure the operative approach. This position requires a flexible neck and may not work for obese or short-necked patients. Other options include the lateral decubitus or three-quarter prone position with the shoulder taped caudally and neck flexed, ensuring the chin is at least two fingerbreadths from the sternum. Regardless of the position chosen, it is generally best to place the vertex parallel to the floor so that the seventh and eighth cranial nerves are inferior relative to the trigeminal nerve, simplifying the approach.37 All pressure points are padded, and an axillary roll is used if necessary. The sitting position can also be used, although it is associated with complications such as air embolism and subdural hematoma.
The arachnoid over the nerve is carefully removed to allow complete inspection of the nerve. Thin, translucent arachnoid can be teased off, but thick, opaque bands must be sharply dissected to avoid injury to the trigeminal nerve, the trochlear nerve (a thin, delicate structure in the arachnoid above the trigeminal nerve), or small vascular branches in the subarachnoid space. Vascular compression is usually seen close to the brainstem, often anterior to the nerve. The site of compression may vary based on symptoms, with symptoms closer to V1 associated with compression of more caudolateral portions of the nerve. The arachnoid anterior to the nerve must be dissected to allow decompression. Endoscopic assistance has been advocated as a method of very thorough evaluation of the nerve with minimal cerebellar retraction.38–41 Some advocate use of a small mirror to visualize behind the nerve.
The artery that most frequently produces compression is the superior cerebellar artery (SCA), which often compresses the nerve anteromedially from within the axilla. Decompression requires elevation of the artery into a horizontal rather than vertical orientation, displacing it upward and away from the nerve. SCA often divides into two branches as it courses around the midbrain, either or both of which may compress the nerve. The anterior inferior cerebellar artery (AICA) may compress the nerve from below, requiring displacement more inferiorly away from the nerve. Sometimes both SCA and AICA are involved, surrounding the nerve like a pincer, in which case both must be mobilized and decompressed. Less commonly, the vertebral or basilar artery contacts the nerve, usually in hypertensive, elderly, and male patients.7 Small arterioles may be seen, more often in younger patients. Compression by a persistent trigeminal artery has also been described.42
Venous contact is frequently seen, often concomitantly with arterial compression. The vein may be anterior (transverse pontine vein), posterior (petrosal vein), or distal at the entrance to Meckel’s cave (trigeminal vein); frequently, compression is caused by one of the many unnamed veins that run along the brainstem.43,44 Posterior venous compression may be obscured by the ridge of the petrous bone. Compression from draining veins from a venous angioma or arteriovenous malformation is sometimes observed. If venous compression is identified, the vein is carefully dissected from the nerve and coagulated with low-voltage small bipolar forceps to prevent spread of current into the adjacent nerve, after which the vein is coagulated and divided.
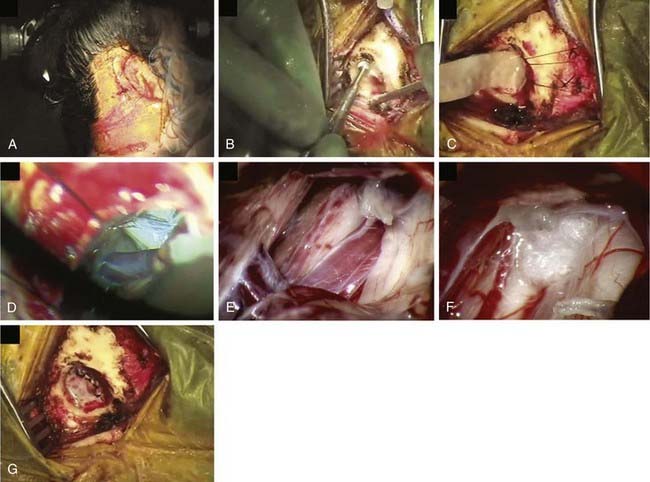
After decompression, the vessels are carefully observed, and topical papaverine-soaked Gelfoam is used if there is any evidence of vasospasm. The operative field is liberally irrigated, retractors are removed, and a Valsalva maneuver is performed by anesthesia to ensure there is no bleeding. The dura is closed in a watertight manner using continuous or interrupted braided 4-0 sutures to prevent subsequent leakage of CSF. It is usually possible to close the dura primarily, but if necessary, a graft of cadaveric, synthetic, or autogenous tissue may be used. Fibrin dural sealant is helpful, especially in redo operations. A piece of Gelfoam is placed over the dura, and a cranioplasty of wire mesh and artificial bone material is fashioned, or the bone is replaced if a craniotomy was performed (Fig. 163-2). The fascia, subcutaneous tissue, and skin are closed in standard fashion using absorbable sutures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree