Fig. 1
Representative hypnograms of wild-type, OX1R −/−, OX2R −/− and double receptor deficient mice over 12 h of the dark phase obtained by concatenating 16 s epoch EEG/EMG stage scores. The height of the horizontal line above baseline indicates the vigilance state of the mouse at the time. Baseline, W represents periods of wakefulness; S non-REM sleep; R REM sleep. Arrowheads highlight direct transitions from wakefulness to REM sleep
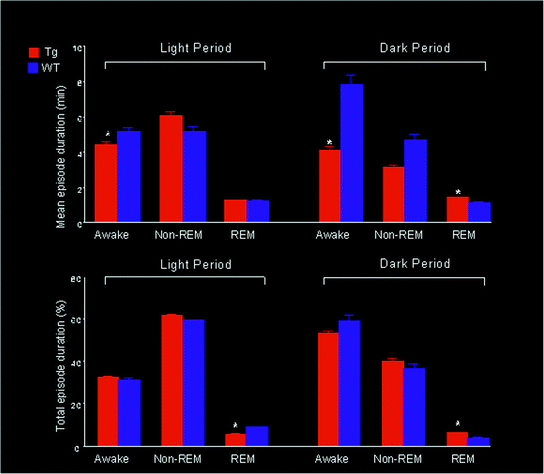
Fig. 2
Durations (upper panels) and total amounts (lower panels) of awake, non-REM sleep, and REM sleep states (means and SEM) for wild type and orexin/ataxin–3 (Tg) mice in light and dark periods. Narcoleptic mice consistently show significantly increased REM sleep times during the dark phase compared to normal mice. *, p < 0.05
2.1 Phenotypes of Narcoleptic Mice
Orexin −/− mice, orexin/ataxin–3 mice, and OX1R/OX2R double orexin receptor deficient (OX1R −/−; OX2R −/−) mice showed the almost identical sleep/wakefulness characteristics, including abrupt behavioral arrest (cataplexy) and fragmentation of sleep/wakefulness states. This section describes these phenotypes (Table 1).
Table 1
Phenotypes of rodent narcolepsy models produced by genetic engineering
Sleep/wake state abnormality | Other phenotypes | Ref. | |
|---|---|---|---|
Prepro-orexin knockout | Cataplexy (+), sleep attacks (+) Sleep/wake fragmentation (severe) | Slight decrease in food intake, mild tendency for obesity (dependent on genetic background) | |
OX1R knockout | Cataplexy (−), sleep attacks (+) Sleep/wake fragmentation | Impairment in emotional memory formation | |
OX2R knockout | Cataplexy (+), sleep attacks (+) Sleep/wake fragmentation (severe) | ND | Willie et al. (2003) |
Orexin/ataxin-3 mouse | Cataplexy (+), sleep attacks (+) Sleep/wake fragmentation (severe) | Decrease in food intake, mild tendency for obesity (dependent on genetic background), lack of food entrainable activity, lack of fasting-induced increase in wake time | Hara et al. (2001) |
Orexin/ataxin-3 rat | Cataplexy (+), sleep attacks (+) Sleep/wake fragmentation (severe) | ND | Beuckmann et al. (2004) |
orexin-tTA; TetO DTA mice | Cataplexy (+) (depending on numbers of orexin neurons ablated), sleep attacks (+) Sleep/wake fragmentation (severe) | Late onset obesity | Tabuchi et al. (2014) |
These phenotypes caused by genetic modifications are strikingly similar to symptomes seen in human narcolepsy. However, it should be noted that familial transmission of human narcolepsy is very rare in humans, and even in these rare cases penetrance is far less than 100 %. No mutation has been found so far either in the prepro–orexin or orexin receptor genes of human narcolepsy patients, except an unusually severe, early onset case, which is associated with mutation in the prepro–orexin gene that impairs peptide trafficking and processing (Peyron et al. 2000).
Behavioral arrests (Cataplexy): These narcoleptic (orexin −/−, orexin/ataxin–3, and OX1R −/− ;OX2R −/−) mice exhibited substantial numbers of behavioral arrests during the dark periods. They were specifically recognized by the abrupt cessation of purposeful motor activity associated with sudden, sustained change in posture that was maintained throughout the episode, ending abruptly with complete resumption of purposeful motor activity. Characteristics of abrupt arrests were very different from those of quiet behavioral states or normal transitions into sleep. Each episode usually lasted for a short period, mostly less than a minute. Occasionally, gait disturbance lasting several seconds was observed immediately before episodes. Side-to-side rocking, without change in overall posture, frequently occurred several seconds after the start of the arrest.
Detailed observations of behaviors during EEG/EMG recordings found that abrupt arrests in narcoleptic mice occurred at timing of the direct transitions from wakefulness to REM sleep or during pre-REM phase immediately after a waking period. (The pre-REM phase shows EEG with high-amplitude spindle oscillations superimposed on non-REM sleep background, and these spindles are observed only during the transition phase immediately prior to REM sleep in wild-type mice.) The direct or very rapid transitions from wakefulness to REM sleep are the most prominent characteristic of EEG/EMG in narcoleptic mice, which were observed almost exclusively in the dark phase, and were never observed in wild-type mice.
Abrupt arrests in orexin −/− mice were ameliorated by systemic administration of clomipramine, an agent used for treatment of human cataplexy. While administration of caffeine, a psychostimulant used to treat excessive sleepiness in human narcolepsy, produce a mild exacerbation of abrupt arrest frequency.
These behavioral, electrophysiological, and pharmacological characteristics of abrupt arrests in narcoleptic mice suggest that these attacks are the counterpart of cataplexy observed in human narcolepsy patients. Cataplexy has been well known to be triggered by strong emotions such as laughing, anger, fear, surprise, and excitement in human narcolepsy patients. In narcoleptic mice, abrupt arrests are very often preceded by purposeful motor activity such as excited ambulation, grooming, burrowing, and climbing. Palatable foods such as chocolates can also trigger cataplexy. The dramatic increase in the number of abrupt arrests noted in the group-housed mice as compared with the individually-housed littermates suggests that social interaction may enhance this attack. Chasing, tail biting, and social grooming were often observed to immediately precede the attacks in the group-housed mice.
Only approximately one-third of human patients experience full loss of muscle tone causing collapse to the floor with the majority having partial cataplexy evidenced by jaw sagging, head bobbing, arm dropping, ptosis, or dysarthria (Parkes et al. 1974). Unambiguous full postural collapse was frequently observed in young orexin −/− mice, while adults tended to collapse onto their ventral surface at odd angles, suggesting some residual muscle tone. Cataplexy is not always instantaneous in human patients but can progress over several seconds with some patients experiencing gait disturbance known as “zig-zag walking”. Similarly, gait ataxia immediately preceding almost one third of abrupt arrests in narcoleptic mice.
Sleepiness and sleep attack: One of the prominent features of sleep/wakefulness patterns in narcoleptic mice was shortened durations of both wakefulness and NREM sleep in the dark phase, causing increased fragmentation of sleep/wake cycle. The shortened durations of wakefulness suggests sleepiness, which is the cardinal symptom of narcolepsy. OX2R −/− mice also showed sleep/wake fragmentation, while occurrence of REM sleep-related abnormalities was very rare as compared to orexin −/−, orexin/ataxin–3 or double orexin receptor knockout mice. This fragmentation was accompanied by statistically insignificant tendency toward reduced amounts of wakefulness and increased amounts of NREM sleep during the dark phase.
Presumptive excessive sleepiness in orexin −/− mice was also shown by detailed analyses of “gradual arrests” in orexin −/− and OX2R −/− mice, which can be interpreted analogous to “sleep attacks” in human narcolepsy. Gradual arrests typically began during quiet wakefulness and could be easily distinguished from the normal onset of resting behavior by lack of stereotypic preparation for sleep (e.g., nesting and/or assumption of a curled or hunched posture, with limbs drawn under the body) and a characteristic ratchet-like “nodding” of the head over a period of several seconds, with a transition to a collapsed posture. Gradual arrests in both orexin −/− and OX2R −/− mice resemble the sleep attacks in human narcolepsy. Unlike cataplexy, the gradual arrests are not associated with strong emotions or muscle atonia, and are reduced by psychostimulants such as amphetamines, modafinil, and caffeine.
Systemic administration of caffeine dose-dependently suppressed gradual arrests, while administration of an anticataplectic agent clomipramine did not affect the frequency of gradual arrests in both orexin −/− and OX2R −/− mice.
EEG/EMG recordings with simultaneous video-capture further differentiated gradual arrests from abrupt ones. As described above, abrupt arrests were accompanied by direct transition from wakefulness to REM sleep in narcoleptic mice. In contrast, EEG/EMG correlates of gradual arrests in narcoleptic mice invariably revealed the onset of attenuated muscle tone, but not atonia, and an EEG transition from wakefulness to NREM sleep. Gradual arrests were occasionally accompanied by apparent automatic behavior, which is continuation of semipurposeful motor activity after the onset of light sleep such as stereotypic chewing of food.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







