Fig. 1
(a) Experimental setup (b) Task sequence (c) Schematic drawing of medial motor areas (top view)
After each animal became proficient in performing this behavioral task, an acrylic recording chamber and head-fixation bolts were implanted on the animal’s skull. After complete recovery from the surgery, we used glass-insulated Elgiloy microelectrodes to make simultaneous recordings of LFPs for the SMA and pre-SMA in one hemisphere while the subjects performed the task (Fig. 1c). Online data collection was performed using a multichannel acquisition processor. Eye position was monitored using an infrared corneal-reflection-monitoring system at 1 kHz (Millennium G200, Matrox).
As a spectral measure of the correlation of the two signals across frequencies, we calculated the coherence from the cross-spectral density between the two LFPs and normalized it by the power spectral density of each [4] using the LFPs recorded in visually guided trials. Coherence values range from 0 to 1. A value of 0 indicates that the two signals were completely uncorrelated, whereas a value of 1 indicates that the signals were completely correlated at frequency f. We defined the frequency that yielded the greatest coherence in the β band (15–40 Hz) as the frequency of interest (f 0) for further analysis.
Our main interest was the cue-dependent modulation of inter-area β oscillations during visually guided trials. We thus defined five consecutive analytic periods in a visually guided trial, as shown at the bottom of Fig. 1b: intertrial period (IT), trial-initiation period (INI), instruction-cue period (INST), motor-preparation period (PREP), and motor-execution period (EXE). To extract the instantaneous phase for the frequency f 0, we applied a wavelet-based approach. The signal of the LFP was convoluted by a complex Gabor’s wavelet w(f 0, t):
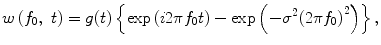
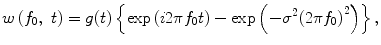
where
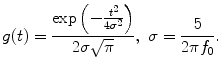
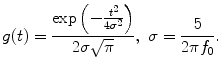
The instantaneous phase difference between a pair of electrodes Δφ(f 0, t) was expressed as the relative phase of the signal recorded from the pre-SMA to that from the SMA:
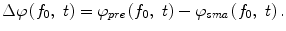
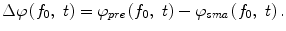
To determine the strength of inter-hemispheric phase synchronization, we calculated a phase-synchronization index (PSI) for a given 500-ms analytic period in a trial as the mean vector length of the angular dispersions of the phase differences in the analytic period [5]. If the phase difference varies little in the analytic period, the PSI is close to 1; otherwise, it is close to 0. To examine how the presentations of the initiation cue and the motor instructional cue influenced the inter-area phase relationship, we computed the mean phase difference 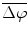 for the IT, INI, and INST periods in each recording session.
for the IT, INI, and INST periods in each recording session. 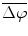 was calculated as the angle of the circular mean of the phase differences observed in the analytic period across trials.
was calculated as the angle of the circular mean of the phase differences observed in the analytic period across trials.
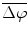 for the IT, INI, and INST periods in each recording session.
for the IT, INI, and INST periods in each recording session. 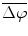 was calculated as the angle of the circular mean of the phase differences observed in the analytic period across trials.
was calculated as the angle of the circular mean of the phase differences observed in the analytic period across trials.3 Results
We performed a total of 30 paired recordings (15 sessions in each hemisphere) for monkey M and 61 (29 and 32 sessions in the left and right hemisphere, respectively) for monkey N. Coherence analysis revealed that f 0 = 35 Hz for monkey M, and f 0 = 22 Hz for monkey N. The dynamics of the inter-area phase difference and the strength of the phase synchronization between areas based on the analysis for the frequency f 0 are discussed in the next section.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






