Molecular Therapy of the Intervertebral Disc
S. Tim Yoon
John Louis-Ugbo
The normal intervertebral disc is a complex avascular fibrocartilaginous structure consisting of a combination chondrocytelike and fibroblastlike cells that support a rich extracellular matrix (3). The cells within the disc synthesize and maintain the matrix. The extracellular matrix provides the mechanical characteristics of the disc. Disc degeneration is characterized by loss of the disc matrix and change in the composition of the matrix of the nucleus to a more fibrotic and less cartilaginous matrix. Disc degeneration is associated with low back pain and other important disease conditions of the spine (1,2). Current treatment options range from pain management to invasive procedures such as spinal fusion and spinal arthroplasty; however, there is no clinically proven biologic therapy of the disc. Although there is much research ongoing on disc therapy, none of the current treatment methods actually treat the disc in a biologic manner. However, there are many different approaches under intense investigation that use biologically active molecules to treat or prevent disc degeneration. This chapter reviews the current status in molecular therapy of the intervertebral disc.
Biology of Intervertebral Disc Degeneration
The disc matrix consists of an elaborate framework of macromolecules that attract and hold water. Collagens and proteoglycans are the primary structural components of the intervertebral disc macromolecular framework. Collagens give the disc tissues their form and strength. The proteoglycans, through their interactions with water, give the tissues stiffness, resistance to compression, and viscoelasticity (4). Table 6.1 shows the profile of the types of collagen and noncollagenous proteins found in the disk. Collagenous proteins are most abundant in the outer annulus, where they comprise 70% of the dry weight, whereas they make up only 20% of the central nucleus pulposus (4). The proteoglycans are present in the greatest concentrations in the central nucleus pulposus, where they comprise 50% of the dry weight of the nucleus in a child (4). The proteoglycan molecule is made up of a core protein to which a variable number of glycosaminoglycan units are covalently attached (5). The most common glycosaminoglycan side chains in the disc are chondroitin sulfate and keratan sulfate, with the former predominating in the normal disc and the latter in the degenerated disc (4,5,6).
To better understand the molecular therapy strategies being investigated for the treatment of the disc, it is important to understand the hallmarks of disc degeneration.
Degenerative processes associated with aging result in morphologic and molecular changes to the disc. Morphologic changes to the aging disc include dehydration, fissuring, and tearing of the nucleus, annulus, and endplates. On the molecular level, degenerative changes may include decreased diffusion of nutrient and waste products, decreased cell viability, accumulation of apoptosis debris, degradative enzyme activity, accumulation of degraded matrix macromolecules, fatigue failure of the matrix, decreased proteoglycan synthesis, and alteration in collagen distribution (4,7).
Degenerative processes associated with aging result in morphologic and molecular changes to the disc. Morphologic changes to the aging disc include dehydration, fissuring, and tearing of the nucleus, annulus, and endplates. On the molecular level, degenerative changes may include decreased diffusion of nutrient and waste products, decreased cell viability, accumulation of apoptosis debris, degradative enzyme activity, accumulation of degraded matrix macromolecules, fatigue failure of the matrix, decreased proteoglycan synthesis, and alteration in collagen distribution (4,7).
TABLE 6.1 Intervertebral Disc Components | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
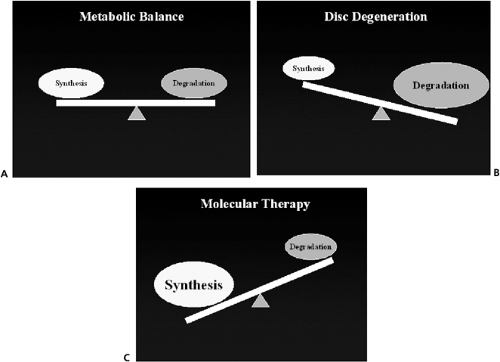
Disc degeneration begins when catabolism and/or the failure to retain matrix proteins consistently exceeds synthesis and/or retention. Decrease in disc nutrition may be an important contributor to disc degeneration. This decrease nutrition may be the result of increased disc size and endplate changes, which when combined with cell density lead to decreased nutrition in the center of the nucleus, low pH, and possibly cell death (4,8). The most prominent change observed includes the progressive loss of proteoglycan, water, and collagen II in the disc matrix of the nucleus pulposus. There are qualitative changes in the matrix that are less well defined including the breakdown of the higher molecular weight proteoglycans and other changes that are more difficult to quantify (e.g., collagen cross-linking, organization of the proteoglycan). One other significant change seems to be the loss of the differentiated chondrocyte phenotype from the nucleus pulposus into a more fibrotic phenotype. Changes in the annulus fibrosus include disorganization of the annular lamella layers and physical defects in the collagenous matrix. Typically, these matrix changes take many years to become apparent and are a result of an imbalance between synthesis and degradation (Fig. 6.1).
Inflammatory mediators have also been identified in the degenerated disc specimens including nitric oxide (NO), interleukin-6 (IL-6), prostaglandin E2 (PGE2), tumor necrosis factor-alpha (TNF-alpha), fibronectin, and matrix metalloproteinases (MMPs) (Table 6.2) (7,9,57,58). However, the pathologic role played by each of these mediators in the disc is not well understood. Insight into the roles played by these mediators is currently being investigated. NO, IL-6, and PGE2 appear to be factors in the inhibition of proteoglycan synthesis, and they are recruited into action by interleukin-1 (IL-1). The proteoglycan matrix also is vulnerable to breakdown in response to IL-1, and this process is thought to be mediated by the MMPs. It seems likely that IL-1 plays a central role in the elaboration of inflammatory mediators, but the nature of that role is not well
defined (12). Seguin et al. (57) recently demonstrated that TNF-alpha, a proinflam-matory cytokine present in herniated nucleus pulposus tissues, at doses of 1 to 5 ng/mL, induced multiple cellular responses including decreased expression of both aggre-can and type II collagen genes; decreases in the accumulation and overall synthesis of
aggrecan and collagen; increased expression of MMP-1, MMP-3, MMP-13, ADAM-TS4, and ADAM-TS5; and induction of ADAM-TS dependent proteoglycan degradation. Within 48 hours, these cellular responses resulted in nucleus pulposus tissue with only 25% of its original proteoglycan content. Based on this result they suggested that TNF-alpha may contribute to the degenerative changes that occur in disc disease.
defined (12). Seguin et al. (57) recently demonstrated that TNF-alpha, a proinflam-matory cytokine present in herniated nucleus pulposus tissues, at doses of 1 to 5 ng/mL, induced multiple cellular responses including decreased expression of both aggre-can and type II collagen genes; decreases in the accumulation and overall synthesis of
aggrecan and collagen; increased expression of MMP-1, MMP-3, MMP-13, ADAM-TS4, and ADAM-TS5; and induction of ADAM-TS dependent proteoglycan degradation. Within 48 hours, these cellular responses resulted in nucleus pulposus tissue with only 25% of its original proteoglycan content. Based on this result they suggested that TNF-alpha may contribute to the degenerative changes that occur in disc disease.
TABLE 6.2 Inflammatory Mediators Implicated in Disc Degeneration | |
|---|---|
|
The goal of molecular therapy is to prevent or reverse these changes in the disc extracellular matrix by altering the balance of degradation to synthesis in favor of synthesis.
Molecular Therapy Strategies for Disc Disease
Therapeutic strategies currently under investigation for the biologic treatment of disc degeneration include the use of cellular components (mesenchymal stem cells, chondrocytes, culture expanded disc cells, disc allograft, etc.), matrix-derivatives [extracellular matrix (ECM)], or molecules that influence disc cell metabolism and phenotype (Table 6.3) (14,15,16,17,18,19,20,21,22,23). This chapter is restricted to the discussion of the molecular intervention in disc degeneration repair.
There are at least four different classes of molecules that are currently being investigated for disc therapy. These include anticatabolics, mitogens, morphogens, and intracellular regulators. Although all of these molecules have some in vitro data, few have been tested in vivo with an animal model of disc degeneration (Table 6.4). Each of these categories is defined and the key literature reviewed in this chapter.
Anticatabolics
Anticatabolics block the activity of degradative enzymes within the disc. Because matrix loss is a balance between matrix synthesis and degradation, it is possible to increase the disc matrix by increasing synthesis or by decreasing degradation. One approach is to prevent matrix loss by inhibiting the degradative enzymes. There are many different catabolic enzymes present in normal disc matrix, but the MMPs make up a particularly important group of catabolic enzymes (9,13). MMPs play an important role in the normal turnover of matrix molecules and are thought to be important in disc degeneration. Their
actions may account for much of the degradation of collagen, aggrecan, versican, and link protein found in the degenerated disc (13). The main members of the MMP family are stromelysin (MMP3), collagenase (MMP1, 8, and 13), and gelatinase (MMP2 and 9). Stromelysin is found mainly in the nucleus pulposus, and it is active in degrading the core protein of proteoglycans. It is the only agent capable of gaining access to the proteolytic cleavage sites, leaving isolated hyaluronate binding regions, degraded proteoglycan aggregates, and glycosaminoglycan fragments as breakdown products (9,10,11,13). Collagenase and gelatinase are more prevalent in the annulus, and they cooperate in the breakdown of collagen. Aggrecan and versican degradation may also result from members of a second family of metalloproteinases, ADAMs. Two members of this family (ADAM-TS4 and 5) show a particular avidity for aggrecan and have been termed aggrecanases (7).
actions may account for much of the degradation of collagen, aggrecan, versican, and link protein found in the degenerated disc (13). The main members of the MMP family are stromelysin (MMP3), collagenase (MMP1, 8, and 13), and gelatinase (MMP2 and 9). Stromelysin is found mainly in the nucleus pulposus, and it is active in degrading the core protein of proteoglycans. It is the only agent capable of gaining access to the proteolytic cleavage sites, leaving isolated hyaluronate binding regions, degraded proteoglycan aggregates, and glycosaminoglycan fragments as breakdown products (9,10,11,13). Collagenase and gelatinase are more prevalent in the annulus, and they cooperate in the breakdown of collagen. Aggrecan and versican degradation may also result from members of a second family of metalloproteinases, ADAMs. Two members of this family (ADAM-TS4 and 5) show a particular avidity for aggrecan and have been termed aggrecanases (7).
TABLE 6.3 Molecular Therapy Strategies for Disc Disease | ||||||||
|---|---|---|---|---|---|---|---|---|
|
TABLE 6.4 Molecules Investigated for Disc Therapy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Within the matrix, MMP activity is normally inhibited by tissue inhibitors of MMPs (TIMPs) (24,25). Wallach et al. (25) successfully delivered an anticatabolic gene, TIMP-I, into cells from degenerated intervertebral discs using an adenoviral vector. They showed an increased expression of TIMP-1 in disc cells and also an increase in the “measured synthesis rate” of proteoglycans. This finding supports catabolic inhibition as a promising avenue of research for the treatment of degenerative disc disease via gene therapy. Another molecule, CPA-926 an esculetin prodrug, that has a better pharmacokinetic profile than esculetin itself has been shown to be anti-inflammatory and antitumorogenic and to prevent degeneration in an osteoarthritic model of cartilage destruction (26). Okuma et al. (26) showed that oral administration of CPA-926 can prevent or delay the onset of disc height loss and demonstrated histologic evidence of disc degeneration in an annulotomy model of disc degeneration in the rabbit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree