Moyamoya Disease
Moyamoya disease (MMD) is a chronic, idiopathic, steno-occlusive arteriopathy affecting the terminal intracranial internal carotid arteries (ICAs) bilaterally. Often, it also affects the proximal segments of the middle cerebral arteries (MCAs) and anterior cerebral arteries (ACAs), and occasionally the proximal posterior cerebral arteries (PCAs). Multiple small basal collateral vascular channels develop at the skull base and in the basal ganglia. These collateral vessels result in the characteristic appearance on angiography from which the name, moyamoya, which loosely translates to “hazy puff of smoke” in Japanese, is derived.1 Moyamoya syndrome (MMS) has clinical and angiographic features identical to those of MMD but develops as a result of a known underlying condition, such as Down syndrome, primordial dwarfism, sickle cell disease, or neurofibromatosis. MMS may also develop following radiation treatment.2 The mainstay therapy for MMD is surgical revascularization of the affected cerebral hemispheres via direct or indirect techniques.
65.1 Pathophysiology
Although MMD was first described in 1957,3 the underlying etiology of its hallmark stenosis remains unknown. There is no evidence of inflammation or atheroma on histologic examination of pathologic specimens.4 MMD vessels demonstrate eccentric fibrocellular thickening of the intima, proliferation of smooth muscle cells in the media, and abnormalities of the internal elastic lamina.4 In response to the cerebral hypoperfusion that is secondary to the steno-occlusive disease, there is increased expression of hypoxia inducible factor 1, vascular endothelial growth factor, and basic fibroblast growth factor.4 The increased expression of these growth factors is thought to promote the development of the multiple small basal collateral vessels. On histopathologic examination, the vessels are thin-walled, with an incomplete internal elastic lamina and reduced numbers of medial smooth muscle cells. The vessels are therefore at increased risk for the development of aneurysmal dilation and rupture, resulting in hemorrhage.4 The staging system of Suzuki and Takaku divides the progression of steno-occlusive disease and collateral development into six sequential stages.1
65.2 Epidemiology
Pediatric MMD is most prevalent in Japan and Korea, with an estimated prevalence of 9 cases per 100,000 children in Japan.5 A recent French study found the incidence of MMD to be 0.065 per 100,000 children per year, with an overall prevalence of 0.39 per 100,000 children.5 MMD has a bimodal age distribution; the first peak occurs in the pediatric population (ages 5 to 9 years), and the second in mid adulthood (ages 45 to 49 years).6 Although its peak incidence is in children is 5 to 9 years of age, MMD can occur in children of any age, with rare cases reported in infancy.7 In children younger than 10 years of age, MMD occurs with equal frequency in boys and girls. In older children, MMD is much more frequent in girls than in boys, with a male-to-female ratio of 2:1.6,8 A family history of MMD is identified in up to 10% of patients.9 Unilateral moyamoya occurs in 27 to 39% of patients with MMD or MMS.8,10,11
65.3 Presentation and Natural History
Most commonly, MMD presents in the pediatric population as a transient ischemic attack (TIA) or stroke. These occur from cerebral hypoperfusion secondary to progressive steno-occlusive disease of the ICA, MCA, and/or ACA. Because of the vascular territories affected, TIA or stroke often manifests as hemiparesis, hemisensory loss, dysarthria, or dysphasia. In some cases, the deficits are nonfocal and involve failure to achieve developmental milestones or a deterioration in school performance. In the series of 143 pediatric patients with MMD and MMS reported by Scott et al, stroke had occurred in 68% and TIA in 43% of patients on presentation. Intracranial hemorrhage is an uncommon presentation of patients with pediatric MMD, occurring in 3 to 9% of patients. This is consistent across studies from Asia and North America.12,13 Intracranial hemorrhage is thought to be secondary to fragility and/or aneurysmal dilation of the basal moyamoya vessels. Deep intracerebral hemorrhage, intraventricular hemorrhage, or both are the most common patterns of bleeding. Subarachnoid hemorrhage has also been reported. Movement disorder in the absence of stroke or hemorrhage is a rare presentation of MMD. This is thought to be due to dilated moyamoya vessels coursing through the basal ganglia.14 These patients may present with hemiballismus or chorea. Headache and seizure are other presenting features reported in MMD. MMS found on the screening images of at-risk patients and incidentally discovered MMD are increasingly common as neuroimaging becomes more frequent and awareness of the disease becomes more widespread.
Although the natural history literature for pediatric MMD is somewhat limited, there appears to be nearly uniform progression of the disease, with a high rate of new clinical events in untreated patients. Imaizumi et al followed 15 untreated pediatric patients with MMD until adulthood. Only 4 patients in the series had a good outcome without limitations to activities of daily living, ongoing TIAs, or headaches.15 In a single-institution series of 35 patients with MMD, 7 of whom were children at the time of diagnosis, Chiu et al reported that the risk for recurrent stroke was 18% in the first year and roughly 5% per year thereafter. Results did not vary with age at presentation.16
Unilateral MMD has a high rate of progression to bilateral disease. In a series of 18 patients with unilateral MMD followed at Stanford, 39% of patients went on to develop disease in the uninvolved side over a mean of 12.7 months.10 In a series of 33 patients with unilateral MMD reported by Smith and Scott, progression to bilateral disease occurred in 30% of patients over a mean of 2.2 years.11 The rate of progression to bilateral disease was more rapid in children younger than 7 years at diagnosis, with a mean time to progression of 0.9 year.11
65.4 Clinical and Radiographic Evaluation
MMD should be considered in children who present with symptoms of permanent or reversible cerebral ischemia, especially if the symptoms are triggered by exertion or hyperventilation. All patients should undergo a thorough neurologic assessment, including a history and physical examination. Information regarding neurologic symptoms should be sought, as should evidence of an underlying condition that could suggest MMS. A detailed neurologic examination is essential in order to provide a baseline that can be followed though the perioperative period. A neuropsychological evaluation can help determine if developmental delay or specific functional disabilities are present.
Magnetic resonance (MR) imaging and MR angiography are the first-line investigations in children undergoing a work-up for possible MMD. FLAIR (fluid-attenuated inversion recovery) sequences effectively demonstrate any areas of old infarct, which are often located in the watershed zones between cerebral vascular territories. On FLAIR and contrast-enhanced T1-weighted images, linear cortical hyperintensity (the “ivy sign”) may be seen that is related to slow flow in cortical vessels.17,18 Diffusion-weighted imaging is sensitive for acute infarction. On T2-weighted sequences, the normal flow voids of the ICA and MCA are absent or diminished, and multiple small flow voids, representing the moyamoya collateral vessels, may be seen in the basal ganglia and thalamus.19
Digital subtraction angiography remains the gold standard technique for diagnosing MMD. It demonstrates stenosis or occlusion of the distal ICA with or without involvement of the proximal ACA or MCA. The collateral moyamoya vessels seen at the base of the brain give the “puff of smoke” appearance on angiography. The Suzuki staging system is based on the angiographic findings.1 For preoperative planning, it is essential that the angiogram include selective external carotid artery (ECA) injections to evaluate the course and caliber of the superficial temporal arteries (STAs).
Perfusion imaging and assessment of the cerebrovascular reserve are an important part of the work-up and surgical decision making for patients with MMD. Multiple modalities are available for assessing cerebral perfusion, including single photon emission computed tomography (SPECT), MR perfusion imaging, BOLD (blood oxygenation level–dependent contrast) MR imaging, CT perfusion imaging, and positron emission tomography (PET). A complete discussion of the attributes of each imaging modality is beyond the scope of this chapter. However, at Stanford, we perform xenon CT or MR perfusion imaging with acetazolamide challenge for the assessment of cerebrovascular reserve in patients with MMD. All patients undergo testing as part of their preoperative work-up and at 6 months postoperatively. Although baseline perfusion imaging is informative, an assessment of the cerebrovascular reserve by means of vasodilator challenge with acetazolamide or hypercapnea provides the best predictor of the territory at risk for stroke. The normal response to vasodilator challenge is an increase in the cerebral blood flow compared with baseline values. In territories with decreased reserve, there is an absence of the normal augmentation of cerebral blood flow or even a steal phenomenon (reduced cerebral blood flow). This occurs because the vessels are already maximally dilated. In patients with MMD, revascularization surgery is recommended for hemispheres in which an absence of normal augmentation or the steal phenomenon is observed, as either of these portends an increased risk for stroke.
65.5 Indications for Surgical Treatment
Considering the high risk for stroke or recurrent stroke in patients with MMD, surgical revascularization should be offered to all patients with MMD who have symptoms of cerebral ischemia. Surgery should also be offered to asymptomatic patients and patients who present with hemorrhage if there is evidence on perfusion imaging of reduced cerebrovascular reserve, which suggests an increased risk for future ischemic events.20 Patients with unilateral MMD in whom progression on the initially unaffected side is noted on follow-up imaging should also be offered treatment if there is evidence of reduced cerebrovascular reserve on perfusion imaging.21
65.6 Surgical Treatment
The surgical treatment for MMD involves revascularization of the affected cerebrovascular territories. This can be accomplished via direct arterial bypass or through indirect revascularization. Direct bypass generally involves anastomosis of an STA to a cortical MCA branch. Other possible donor vessels include the middle meningeal artery (MMA) and the occipital artery, if there is no useable STA. Indirect bypass involves the application of vascularized tissue to the cortical surface of the brain after it has been stripped of arachnoid in order to facilitate the development of collateral blood supply. Commonly used techniques include encephalo-duro-arterial-synangiosis (EDAS) and encephalo-myo-synangiosis (EMS). Other techniques include dural inversion, onlay of pericranium, multiple bur holes, and free or pedicled omental transposition. In the Stanford series of pediatric patients with MMD, 162 hemispheres were revascularized in 96 patients. A direct bypass was performed in 67% of the patients, and an indirect bypass was performed in the remaining 33%. The determining factors precluding direct bypass were size and fragility of the STA or M4 segment in all cases. Indirect procedures were more common in younger children. The youngest child in whom a direct bypass was feasible was 4.3 years old.8
We use direct revascularization as often as possible because the immediate benefit of augmentation of blood flow to the affected territory potentially reduces the risk for perioperative infarction. If a direct bypass cannot be performed, generally because of the inadequate caliber of available donor or recipient vessels, we perform an EDAS.
65.7 Perioperative Care and Anesthesia Considerations
The major concern in the surgical management of MMD is the prevention of perioperative strokes. During the immediate preoperative period, patients should be maintained on antiplatelet therapy, and adequate hydration should be ensured. There is some evidence that hyperventilation (or iatrogenic hypocapnea) may precipitate strokes in patients with MMD. Therefore, it is imperative that painful and fear-provoking stimuli be minimized to avoid triggering inconsolable crying before the induction of anesthesia. During the procedure, iatrogenic hyperventilation must be avoided. This is detected by monitoring end-tidal carbon dioxide or frequently measuring arterial blood gases. Throughout the perioperative and intraoperative period, careful monitoring of the blood pressure is essential, and target pressures should be at or above the patient’s baseline blood pressure. After induction, all patients have an arterial line inserted, and most have a central venous catheter placed for intraoperative and postoperative monitoring and management. Intraoperative neuromonitoring, including somatosensory and motor evoked potentials and electroencephalography (EEG), is always used. All changes in potentials are communicated to the anesthesia and surgical team immediately to allow optimization of the cerebral perfusion through maneuvers like elevation of the blood pressure. Mild intraoperative hypothermia, with a goal temperature of 33°C, is employed in all cases. Although there is no rigorous clinical evidence to support the use of hypothermia for focal stroke, theoretical benefits of neuroprotection exist, prospective randomized studies have proved its benefit for global stroke after cardiac arrest and neonatal hypoxic–ischemic encephalopathy,22–25 and we have found it to be safe.26 In cases of direct bypass, the mean arterial pressure is raised to 10 mm Hg above baseline, and EEG burst suppression is achieved with the use of propofol during the temporary occlusion of the recipient M4 branch.
65.8 Direct Revascularization with the Superficial Temporal Artery–Middle Cerebral Artery Bypass
For an STA–MCA bypass to be feasible, the donor and recipient vessels must be of sufficient caliber to allow creation of the anastomosis. The minimum caliber of the donor and recipient vessels that we anastomose is 0.6 mm, but vessels should ideally have a diameter of more than 1.0 mm. The donor vessel is chosen preoperatively based on the ECA injection of the angiogram, and the larger STA branch is used. In cases in which the STA branches are similar in caliber, we preferentially use the parietal branch because it is easier to harvest and the incision is more cosmetic. The recipient vessel is chosen intraoperatively based on direct inspection of the exposed M4 vessels.
Following the induction of anesthesia, the patient is positioned supine with a lift under the shoulder and the head turned contralaterally, so that the sagittal plane of the head is parallel to the floor. The Mayfield (Integra, Plainsboro, NJ) three-point fixation system is used in patients undergoing direct bypass to ensure stability of the head during the microanastomosis. The surface projection of the donor STA branch is marked on the scalp with a “pencil” Doppler to identify its course. We prefer to use the parietal branch of the STA. The incision line is marked immediately overlying the STA, with the proximal end anterior to the tragus of the ear. If the frontal branch of the STA is being used, the incision is kept just behind the hairline, and the distal end is curved posteriorly to allow adequate exposure of the perisylvian region with the craniotomy. Minimal or no hair is clipped for these cases.
The donor vessel is harvested in a proximal-to-distal direction, with the operating microscope used for magnification. The overlying scalp is incised with a scalpel and standard microsurgical techniques. Littler scissors and bipolar cautery are used to isolate the STA. The vessel is isolated circumferentially, with a 4- to 6-mm cuff of fascia on either side of the vessel. Small branches are coagulated and cut, and larger branches are tied off with 4–0 ties. A length of at least 7 cm of STA must be isolated to facilitate performance of the anastomosis. At the proximal end, a short segment of the vessel is denuded of soft tissue to allow temporary clipping and flow measurements. A 1-cm-long segment of the distal vessel is also denuded of soft tissue to facilitate the anastomosis. The STA is left in continuity until immediately before the anastomosis is performed to minimize the risk for vessel thrombosis and also to allow conversion to an EDAS if a suitable recipient vessel is not available ▶ Fig. 65.1.
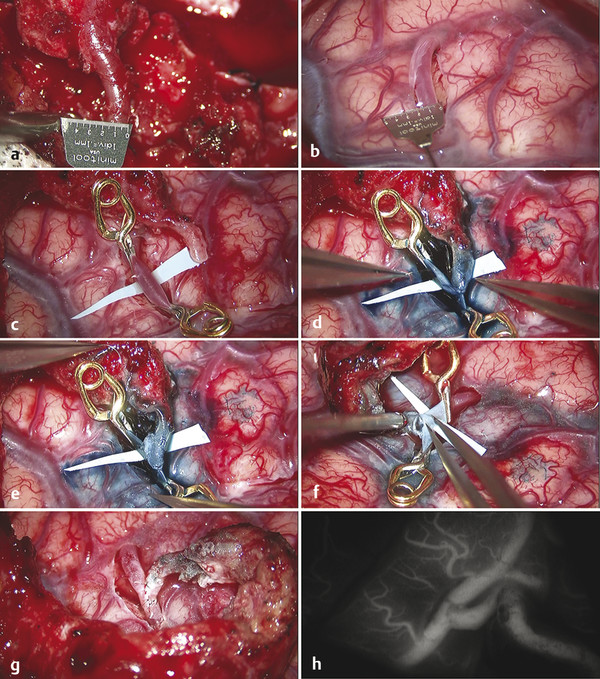
Fig. 65.1 Superficial temporal artery to middle cerebral artery (STA–MCA) bypass for moyamoya disease. (a) The minimum diameter of the distal STA or M4 for completion of an anastomosis is 0.6 mm. Ideally, the vessel diameter should be at least 1 mm. (b) After arachnoid dissection has exposed the M4 branch to be used as the recipient vessel, it is also measured to ensure adequate diameter. (c) Specially designed Anspach-Lazic (Peter Lazic GmbH, Tuttlingen, Germany) 3-mm temporary aneurysm clips are used for temporary occlusion of the recipient M4 branch. These clips have a narrower profile and smaller size than those of standard temporary aneurysm clips, which facilitate completion of the anastomosis. (d) After an elliptical arteriotomy is made in the recipient vessel, the toe of the fish-mouthed STA is sutured in place with 10–0 Monosof (Covidien, Mansfield, MA) sutures. (e) Next, the heel of the STA is sutured. This order allows tailoring of the fish mouth of the STA if there is a mismatch between the donor and recipient vessels. The side walls of the anastomosis are then sutured with interrupted 10–0 Monosof sutures. (f) After completion of the first side wall, the insides of the vessels are checked to ensure that the back wall has not been caught in a suture. (g) The completed anastomosis, with the redundant STA and fascial cuff lying on the cortical surface to allow the further development of indirect collaterals in addition to the direct bypass. (h) Indocyanine green (ICG) video angiography is used to confirm flow through the bypass.









