Chapter 14 Mucosal Disease of the Head and Neck
ANATOMY
Nasopharynx
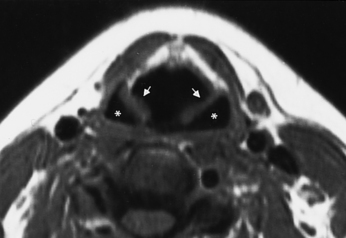
The nasopharynx is broadly defined as that area of the mucosal surface that encompasses the superior, lateral, and posterior walls of the aerodigestive tract, above the soft and hard palate, extending to the skull base. Below the nasopharynx lies the oral cavity anteriorly and the oropharynx posteriorly. The nasopharynx is lined by stratified squamous and ciliated columnar epithelium and includes the mucosa overlying the eustachian tube orifice, the cartilaginous portion of the eustachian tube (torus tubarius), and the posterolateral pharyngeal recesses known as the fossa of Rosenmüller (Fig. 14-1). The mucosa of the nasopharynx is separated from the deeper retropharyngeal space by the pharyngobasilar fascia. The pharyngobasilar fascia forms a rather stiff barrier to the spread of mucosal diseases, but it has bilateral openings, the sinus of Morgagni, to emit the eustachian tubes. The buccopharyngeal fascia is deep to the pharyngobasilar fascia and serves as another of the fascial barriers from nasopharynx to retropharyngeal and parapharyngeal spaces.
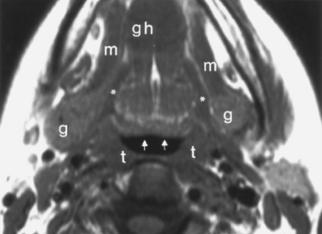
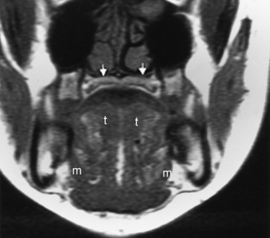
On either side of the eustachian tube orifice lie, anterolaterally, the tensor veli palatini muscle (innervated by cranial nerve V-3) and posteromedially the levator veli palatini muscle (innervated by cranial nerve X), deep to the mucosa (see Fig. 14-1). These muscles elevate and tense the soft palate, into which they insert, preventing nasal regurgitation during swallowing. Between these muscles is a slip of fat (typically obliterated in early nasopharyngeal carcinomas), and posterolateral to these muscles lies the fat-filled parapharyngeal space. Fixate on fat—an effective friend for finding pharyngeal foulness.
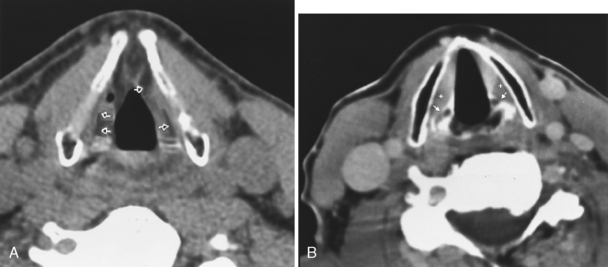
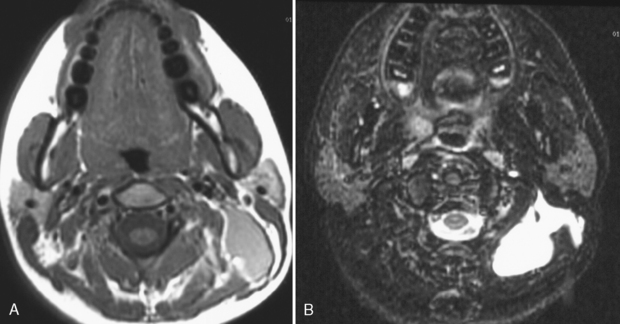
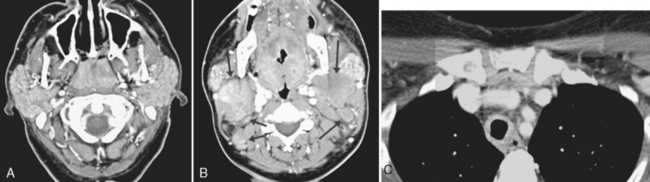
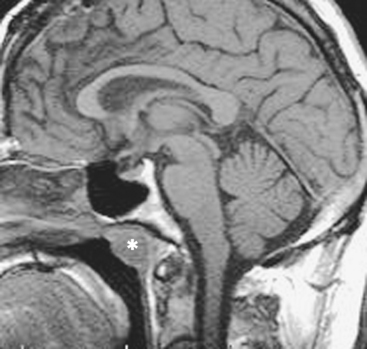
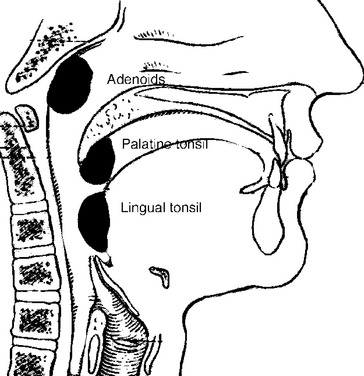
The nasopharynx also houses the adenoidal lymphoid tissue. The amount of adenoidal tissue present depends on the age of the patient, because it atrophies by the fourth decade of life. In a young adult the normal adenoidal tissue or lymphoid hyperplasia may simulate a lymphoma or an exophytic squamous cell carcinoma. In human immunodeficiency virus (HIV)-positive patients, on average, the width of the adenoidal tissue is twice that of age-matched controls (Fig. 14-2). The normal variation in adenoidal thickness requires vigilance for deep invasion, infiltration of the parapharyngeal fat, concomitant middle ear or mastoid opacification, skull base erosion, or obscuration of the planes between the tensor and levator veli palatini muscles to definitively suggest carcinoma. Lymphoid tissue here and in the palatine and lingual tonsils is usually slightly hyperintense on T1-weighted images (T1WI) and hyperintense on T2-weighted images (T2WI) and enhances. These imaging characteristics would be unusual for a squamous cell carcinoma but could occur in a lymphoma. The adenoids, the palatine (also known as faucial) tonsils, and the lingual tonsils make up Waldeyer’s ring of lymphoid tissue (Fig. 14-3). All of these regions may show lymphoid hyperplasia in cases of HIV infection and mononucleosis or because of exposure to chronic irritants (cigarette smoke, alcohol, and chewing tobacco).
Oropharynx
The oropharynx includes the posterior third of the tongue (also known as the tongue base), the vallecula, the palatine tonsils and tonsillar fossa, the posterior and superior pharyngeal walls from the level of the soft palate down to the pharyngoepiglottic folds, the uvula, and the soft palate (Fig. 14-4). The circumvallate papillae of the tongue separate the oral tongue (a part of the oral cavity) anteriorly from the oropharynx posteriorly. The hard palate is part of the oral cavity, but the soft palate is part of the oropharynx. Besides the palatine tonsils, the oropharynx also contains the lingual tonsillar tissue seen at the base of the tongue.
Oral Cavity
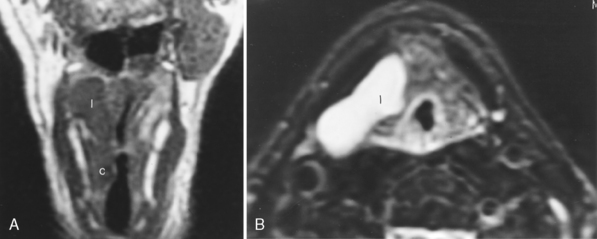
For the radiologist the phrase “the floor of the mouth” should be equated with the mylohyoid musculature (which constitutes the inferior sling of the mouth) and the sublingual space, between the mylohyoid muscle and the hyoglossus muscle (Fig. 14-5). The lingual nerve from the trigeminal nerve and the hypoglossal nerve run together from the floor of the mouth into the tongue base and sublingual space and are important for the surgeon to identify and retain to maintain tongue function. Radiologists must identify whether tumor is in the sublingual space to alert the surgeon regarding the potential for invasion or sacrifice of these nerves. The chorda tympani from the facial nerve supplies taste to the anterior two thirds of the tongue, and its branches join those of the lingual nerve. The glossopharyngeal nerve supplies taste to the posterior third of the tongue.
Hypopharynx
The hypopharynx is the junction between pharynx and larynx and includes three major subsites: the piriform sinus, the postcricoid region (pharyngoesophageal junction), and the posterior pharyngeal wall above the inferior border of the cricoid cartilage (Fig. 14-6). The top of the hypopharynx is at the epiglottic level, and its inferior border is the pharyngoesophageal junction. The mucosa over the posterior surface of the cricoarytenoid joints is part of the hypopharynx. The anteromedial border of the piriform sinus is the aryepiglottic fold, a structure of the supraglottic larynx.
Larynx
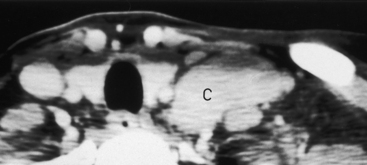
The larynx is broadly separated into the supraglottis, the glottis, and the subglottis (Fig. 14-7). Each of these areas is dealt with individually by the head and neck oncologic surgeon, although lesions often cross these boundaries of the larynx (transglottic cancers). The supraglottis includes the false vocal cords, the arytenoids, the epiglottis, and the aryepiglottic folds. The glottis includes the true vocal cords, the anterior and posterior commissures, and the vocal ligament extending from the arytenoid cartilage. The laryngeal ventricle is said to separate the supraglottis and glottis, but is itself a part of the supraglottis. The subglottis begins 1 cm below the ventricle and extends to the first tracheal ring.
The larynx is anchored on a framework composed of the hyoid bone, the epiglottis, the thyroid cartilage, the cricoid cartilage, and the arytenoids, each of which has an integral function. Of these, the complete ring of the cricoid cartilage is the only indispensable strut for preservation of airway patency. The major role of the epiglottis is to protect the airway during swallowing. From the inferior portion of the arytenoid cartilage the vocal ligament stretches to the thyroid cartilage anteriorly and supports the vocal cord. The lower cricoarytenoid joint is the marker for the level of the true vocal cord (Fig. 14-8). The true vocal cords meet in the midline at the anterior commissure. This junction should be no more than 1 to 2 mm thick. The posterior commissure refers to the mucosa between the two vocal processes on the anterior surface of the arytenoid cartilage.
Lymph Nodes
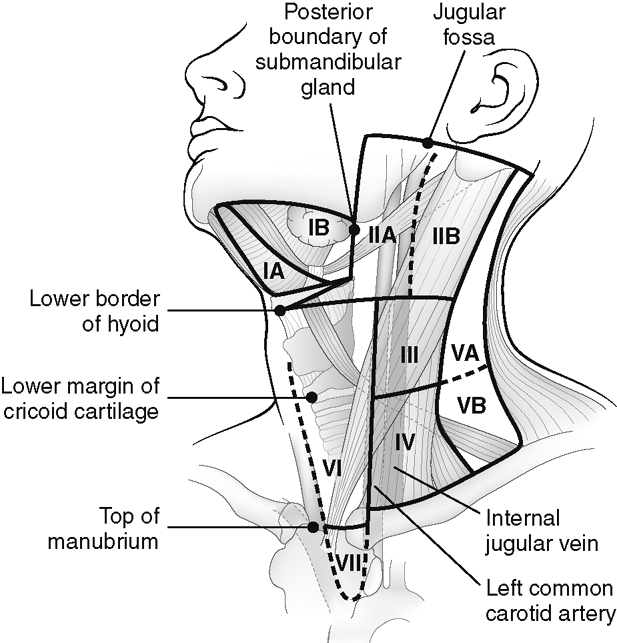
The nomenclature of the lymph nodes of the neck has undergone a recent change, which is supported by various societies of head and neck surgeons. This nomenclature identifies the lymph nodes by a grading system from I to VII (Fig. 14-9). Level I lymph nodes include the submental (IA) and submandibular (IB) lymph node chains. Level II lymph nodes include the internal jugular lymph node chain above the hyoid bone, which are anterior or immediately adjacent to the jugular vein (IIA) or posterior to the jugular vein deep to the sternocleidomastoid muscle (IIB). The level IIA nodes include the jugulodigastric node, a node that sits along the posterior belly of the digastric muscle at the upper pharyngeal level. Level III lymph nodes involve the jugular chain between the hyoid and cricoid cartilage, and level IV lymph nodes involve the jugular chain below the cricoid cartilage. Level V is designated as all the lymph nodes of the posterior triangle of the neck (deep and posterior to the sternocleidomastoid muscle and above the clavicle). If above the cricoids, they are designated VA; if below, VB. Level VI lymph nodes are those that previously were identified in the anterior jugular and visceral chain in front of the thyroid gland. Finally, level VII nodes are in the superior mediastinum region.
CONGENITAL LESIONS OF THE MUCOSA
Branchial Apparatus Fistulae
Branchial cleft cysts (BCCs) are not considered to be “mucosal lesions” so they are discussed in Chapter 15. However, when they drain to the mucosal surface, they may present as such. Second branchial cleft fistulae may drain to the palatine tonsil from their typical location deep to the sternocleidomastoid muscle but superficial to the carotid sheath (Bailey type II, second BCC). Those arising in the parapharyngeal space and potentially draining to the mucosa are classified as Bailey type IV, second BCC.
The piriform sinus is a drainage site for third BCCs, and the piriform sinus apex may be a site of sinus tracts leading from fourth BCCs (see Table 15-2 and Fig. 15-59). The third branchial cleft sinus tract passes between the common carotid artery and vagus nerve to the anterior border of the inferior sternocleidomastoid muscle. The fourth branchial cleft sinus tract passes around the great vessels and the aortic arch on the left side. Piriform sinus fistulae from the fourth branchial apparatus may pass to the thyroid gland, causing acute suppurative thyroiditis or open to the skin. The vast majority of these fistulae are left sided. Recurrence rates are about 40%. Most occur in children.
Cephaloceles
Transsphenoidal encephaloceles may present as a nasopharyngeal mass. Usually the endoscopist is able to identify dura, dural blood vessels, or pulsating brain tissue. This entity is seen most commonly in Southeast Asians (see Fig. 9-19). Encephaloceles often include unexpected anatomy of intracranial origin. Teratomas tend to transgress transsphenoidally too and transmit totipotential, “toti-intensity” tissue.
The craniopharyngeal canal is the route by which the anterior pituitary gland ascends from the pharynx to the pituitary fossa. Nasopharyngeal craniopharyngiomas are very uncommon but represent neoplasms related to the craniopharyngeal duct that ascends from the pharynx to the suprasellar region. They are mentioned here in the congenital section for confusion purposes and also in Chapter 11.
Developmental Inclusion Cysts
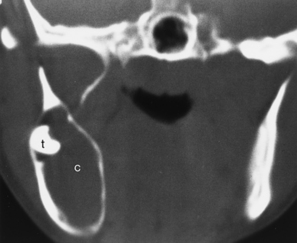
Epidermoids and less commonly dermoids and lymphangiomas (usually multilocular) may occur in the tongue, usually in its oral portion. The dermoids contain fat or fluid and are usually in the floor of the mouth. Epidermoid cysts usually occur in the sublingual space and have fluid density and signal intensity (Fig. 14-10). The lesion may have geometric designs within the cyst.
Lingual Thyroid Gland
Thyroid tissue may be seen embedded in the posterior tongue because of arrested descent from the foramen cecum, which is located at the junction of the two sides of the circumvallate papillae along the anterior edge of the base of the tongue. This thyroid tissue is typically in the midline, hyperdense on noncontrast CT because of its natural iodine content, and enhancing. After identifying the lingual thyroid tissue, the radiologist must next image the neck for any additional thyroid gland residua (Fig. 14-11). In 80% of cases the lingual thyroid tissue is the only functioning thyroid tissue in the body. Although treatment is excisional due to a risk of airway compromise in adolescence, the surgeon may elect to leave a little length of lasting lingual thyroid in place if this is the case. Incomplete descent of the thyroid gland to its normal location in the lower part of the neck is an uncommon congenital anomaly.
Lymphatic Vascular Malformations
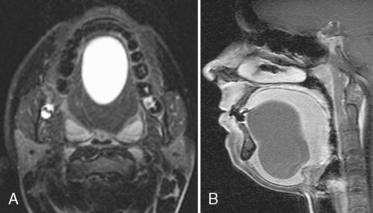
Congenital lymphatic lesions of the head and neck (in decreasing size) include cystic hygromas, cavernous lymphangiomas, and capillary lymphangiomas. Most cystic hygromas are apparent at birth (50% to 60%) and are seen most commonly in the neck (75%) and axilla (20%) (Fig. 14-12). Cystic hygromas may transilluminate. Occasionally, they may be diagnosed in utero because of polyhydramnios. They are usually hypodense and multiloculated on CT and have variable intensity on T1WI and T2WI because of their potential for proteinaceous-chylous-hemorrhagic content. The mass may present acutely as a result of spontaneous or posttraumatic intralesional hemorrhage with fluid-fluid levels or infection. The cystic hygroma is not invasive but is infiltrative, being compressible and distorted by arteries. The cause of these lesions is thought to be obstruction of the primitive lymphatic channels that are derived from the venous system early in gestation. An association with Turner syndrome, Noonan syndrome, and fetal alcohol syndrome is well-described.
Thyroglossal Duct Cysts
Thyroglossal duct cysts (TGDCs) may also occur in the tongue base or floor of mouth. TGDCs are much more common entities than lingual thyroid glands but are usually infrahyoidal. This entity is covered in the thyroid gland section of Chapter 15.
Tornwaldt Cyst
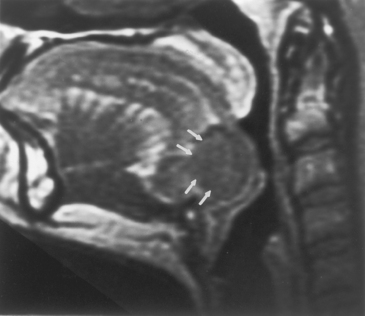
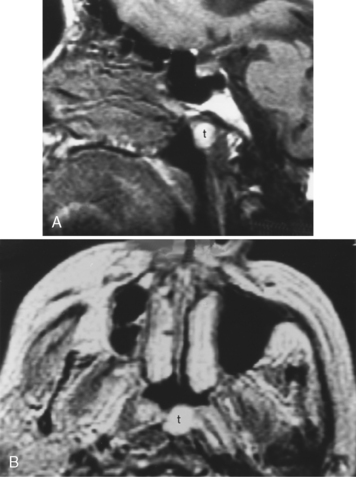
The most common congenital lesion of the nasopharynx (and by the way, of the head and neck) is the Tornwaldt cyst (Fig. 14-13). This results from apposition of the mucosal surfaces of the nasopharynx in the midline as the notochord ascends through the clivus to create the neural plate. A Tornwaldt cyst usually is hypodense on CT. The intensity on magnetic resonance (MR) imaging varies with the protein content but is usually bright on T1WI and T2WI. The cyst is usually well-defined and characteristically occurs in the midline, although it may be seen off-midline in a small percentage of cases. The cysts become infected on rare occasions and may be a source of persistent halitosis.
INFLAMMATORY LESIONS OF THE AERODIGESTIVE SYSTEM MUCOSA (BOX 14-1)
Adenitis
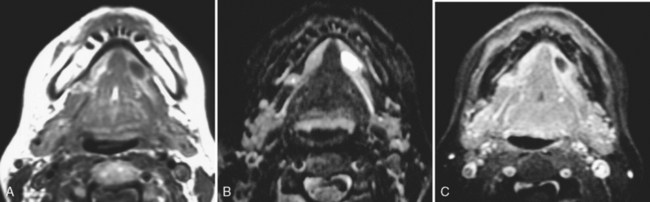
Children seem to have a predilection for dramatic adenitis. Posterior triangle reactive adenopathy often accompanies the ubiquitous middle ear infections of young childhood. Pharyngitis can lead to complications of retropharyngitis, now recognized as an inflammatory process usually mediated by retropharyngeal adenitis, not direct spread (Fig. 14-14). Nonetheless, it can be a fulminant infection that can require surgical intervention.
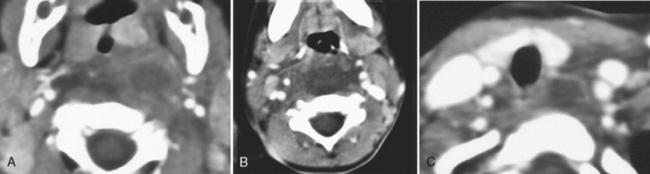
Figure 14-14 Retropharyngeal adenitis. A, A retropharyngeal low-density collection likely represents necrotizing adenitis from the antecedent pharyngitis. Note the low density in the retropharyngeal space away from the node that may be due to either edema or phlegmon. B, Spread inferiorly from the nasopharyngeal level can occur due to the potential space afforded by the retropharyngeal fat and “danger space.” (Ah! but that is better left for a discussion in Chapter 15!) C, Spreading all the way down to the tracheal level—and beyond—is possible.
Castleman Disease
Castleman disease (angiofollicular hyperplasia) is a nodal disease that can be seen in the chest (70% of cases) and the head and neck (10%). Usually the patient is asymptomatic and younger than age 30 years. The unique feature of these nodes is their avid enhancement because of hypervascular stroma (Fig. 14-15). The nodes are nonnecrotic. There may be a stellate area of nonenhancement in the center of the enhancing nodes.
Mononucleosis
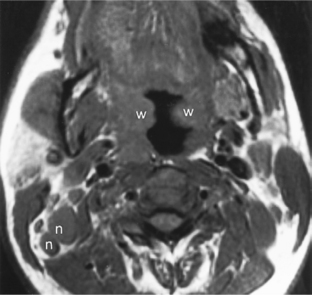
Mononucleosis, caused by Epstein-Barr virus (EBV) infection, is another inflammatory source of multiple enlarged nonnecrotic lymph nodes. The differential diagnosis includes acquired immune deficiency syndrome (AIDS) and sarcoidosis. In AIDS and mononucleosis the lymphoid tissue of Waldeyer’s ring (adenoids, palatine tonsils, and lingual tonsils) may be hypertrophied, and bilateral nodes are the rule (Fig. 14-16). If intraparotid lymphoepithelial cysts are present, HIV infection is more likely. If the parotid glands are enlarged and infiltrated diffusely, sarcoidosis may be suggested, but a chest radiograph with bihilar adenopathy may be the best clue. Otherwise the differential diagnosis may rely on serology or the appropriate history.
Tuberculous Adenitis
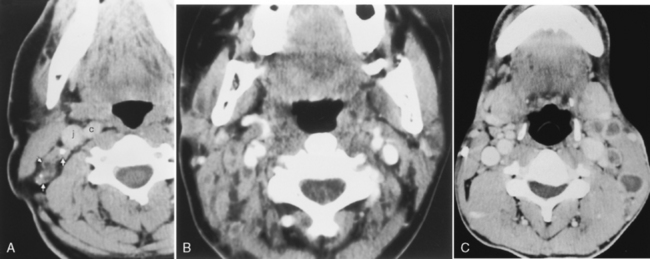
The classic cause of an inflammatory cervical adenitis is tuberculous adenitis (scrofula), usually seen in Southeast Asians. The patients have painless posterior neck masses with or without systemic symptoms. The source of the infection is usually contaminated milk associated with Mycobacterium bovis, causing a subclinical pharyngitis. In the United States Mycobacterium tuberculosis is the most common cause. Atypical mycobacteria (Mycobacterium scrofulaceum especially) may also cause tuberculous adenitis. Concomitant pulmonary tuberculosis is relatively uncommon. The disease manifests as bilateral low-density necrotic lymph nodes, often in the level V posterior triangle distribution (Fig. 14-17). The nodes often have ringlike thick enhancement and appear multiloculated. Adjacent fat planes are obscured or edematous. The nodes often calcify after treatment (Box 14-2). The differential diagnosis of calcified nodes should include tuberculosis, other granulomatous diseases (fungi, sarcoidosis, and Thorotrast granulomas), treated lymphoma, anthrosilicosis, and metastatic thyroid carcinoma, adenocarcinoma, or squamous carcinoma.
Croup
Epiglottitis must be distinguished from croup, a viral infection that occurs in children younger than those affected by epiglottitis. Parainfluenza 1 or 2 and influenza A are the most common pathogens. Croup is characterized by edema of the glottis and subglottis such that the normal contours of the laryngeal ventricle and true cords are obliterated, and a smooth steeple-shaped laryngeal airway is produced. Croup is a much less morbid disease than epiglottitis and should be distinguishable on the basis of plain films (Table 14-1). Woof, woof—sounds like the classic barking cough of croup.
Table 14-1 Differentiation of Croup from Epiglottitis
| Feature | Croup | Epiglottitis |
|---|---|---|
| Organism | Parainfluenza virus | Haemophilus influenzae (children); streptococcus (adults) |
| Radiographic findings | Steeple-shaped subglottis | Thickened epiglottis, arytenoids, and aryepiglottic folds |
| Region of larynx | Glottic/subglottic | Supraglottic |
| Fever | No | Yes |
| Age at onset (yr) | <2 | >2 |
| Dysphagia | No | Yes |
Macroglossia
Inflammation/edema of the tongue may occur with acute allergic reactions and is one of the complications of iodinated contrast injections of which all radiologists should be aware. Treatment is dependent on severity and airway compromise but have the EpiPen, Benadryl, and steroids ready. Diffuse macroglossia may be present in patients with hypothyroidism, amyloidosis, Down syndrome, glycogen storage diseases, mucopolysaccaridoses, and neurofibromatosis, among other conditions (Box 14-3 and Fig. 14-18).
Odontogenic Lesions
Numerous odontogenic abnormalities may be found within the oral cavity, the most common of which is the lytic lesion known as the radicular (periapical) cyst (Table 14-2). This is a lucent lesion of either the mandible or the maxilla and is associated with an infected tooth. These lesions rarely come to the medical radiologist; usually the dentist diagnoses them on bite-wing films. The second most common odontogenic cystic lesion is the dentigerous cyst. This cyst is associated with an unerupted tooth and is usually seen in the mandible, particularly around the molar region. Both the radicular cyst and the dentigerous cyst are usually unilocular, as opposed to the keratocyst and the ameloblastoma, which are benign but aggressive multilocular cystic lesions most commonly affecting the mandible (Fig. 14-19). Keratocysts are associated with the basal cell nevus (Gorlin) syndrome, in which patients have proliferative falcine calcification, multiple basal cell carcinomas of the skin, scoliosis, ribbon-shaped ribs, central nervous system tumors, and keratocysts of the mandible.
Table 14-2 Benign Lytic Dental Lesions
| Lesion | Imaging Appearance | Typical Clinical Findings |
|---|---|---|
| Ameloblastoma | Multiloculated (60% of cases) lytic lesion often associated with dentigerous cysts; hyperostotic margins; cortex eroded or penetrated | 85% in mandibular molar area with expanded jaw; painless, male predominance |
| Brown tumor | Lytic lesion with erosion of lamina dura; ill-defined borders | Hyperparathyroidism |
| Central odontogenic fibroma | Multilocular lesion with sclerotic borders | Expanded mandible |
| Cherubism | Bilateral, symmetric multilocular lucencies (soap bubble) in posterior mandible; expanded cortex without perforation; simulates fibrous dysplasia | Painless; bilateral enlargement of lower part of face; angelic appearance; autosomal dominant inheritance; regression after adolescence |
| Dentigerous (follicular) cyst | Lytic, lucent, expansile lesion adjacent to unerupted tooth; spares cortex; sclerotic margins | Unerupted asymptomatic third molar or canine tooth |
| Giant cell granuloma | Well-defined multilocular lucency with sclerotic margins involving mandible | Asymptomatic in children and young adults |
| Globulomaxillary cyst | Lucent lesion between lateral incisor and canine in maxilla | Asymptomatic |
| Incisive canal cyst (nasopalatine duct cyst) | Lucent lesion in midline hard palate with hyperostotic borders at canal | Swollen anterior hard palate; asymptomatic |
| Keratocyst (primordial cyst) | Unilocular or multilocular expansile lucent lesion; erodes cortex but does not perforate it | Recurrent posterior mandibular lesion with thin walls; associated with basal cell nevus syndrome |
| Radicular (periapical) cyst | Lytic; lucent at apex of erupted tooth; loss of lamina dura; hyperostotic borders | Carious, tender nonvital tooth |
Sclerotic dental lesions also span the spectrum of inflammatory, benign neoplastic, and malignant lesions (Table 14-3). Dental disease is daunting due to dumbfounding and duplicative distributions and depictions of different dense and destructive diagnoses.
Table 14-3 Sclerotic Dental Lesions
| Lesion | Imaging Appearance | Typical Clinical Findings |
|---|---|---|
| Adenomatoid odontogenic tumor | Calcified well-defined lesions with thick capsule; associated with impacted tooth; involves crown of tooth | Teenagers with impacted maxillary front teeth; painless; female predominance |
| Cementoblastoma | Circular radiodensity attached to a mandibular tooth with pencil-thin border; surrounded by lucency; radial spicules | Expanded mandible with vital tooth; occurs in first through third decades of life |
| Chondrosarcoma | Moth-eaten appearance with chondroid whorls; may be lucent or dense | Maxillary swelling; painful in adults |
| Ewing’s sarcoma | Onion-skinning; destructive lesion; poorly defined | Age 5–25 yr with painful mandibular mass, fever, rapid growth, loose teeth |
| Fibrous dysplasia | Ground-glass appearance, homogeneous in later stages | Focal painless mass; slow growth; posterior maxilla |
| Garré’s sclerosing osteomyelitis | Predominantly sclerotic bony lestion; hot on scintigrams; often with periosteal reaction | Bony-hard cortical swelling of mandible; carious molars; nonvital teeth and apical lucency |
| Lymphoma | “Moth-eaten,” sclerotic bone | Often systemic symptoms |
| Metastases | Dense permeative lesions | Lung, breast, prostate, colon, kidney, thyroid primary tumors; loose teeth; often painful |
| Odontoma | Young patient with mass between canines or in mandible; painless; young adults or children | |
| Osteoma | Dense benign excresence; well-defined | Associated with Gardner syndrome with colonic polyps, supernumerary teeth, cysts; seen as a torus on palate; painless, slow-growing |
| Osteosarcoma | Sclerotic or lytic; poorly defined with opaque spicules with sunray appearance; resorbs roots | Maxillary or mandibular mass; rapid growth; painful; loose teeth |
| Paget disease | Dense thickened bone with risk of osteosarcoma; cotton-wool appearance in maxilla; loss of lamina dura | Elderly patient with dentures no longer fitting; commonly in maxilla |
| Pindborg tumor | Multiple small calcifications within lytic lesion associated with impacted teeth | Mass in mandibular molar |
Supraglottitis
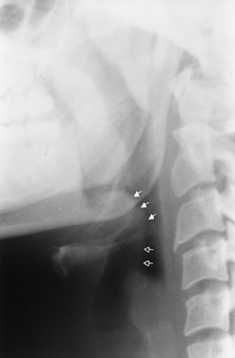
We use the term supraglottitis because most cases of “epiglottitis” will affect the aryepiglottic folds and even the superior aspects of the arytenoids. In some cases the soft palate and prevertebral swelling may be the predominant factor in creating upper respiratory symptoms. Epiglottitis is a life-threatening illness that generally is seen in 2- to 4-year-old children. This is a bacterial infection of the epiglottis that is usually caused by Haemophilus influenzae. The epiglottis is markedly thickened (thumb-shaped), and dilation of the airway above the epiglottis is present. Manipulation of the epiglottis in the acute setting may cause diffuse laryngeal edema, producing acute respiratory compromise. Fifty percent of infants with this disorder ultimately require intubation. Epiglottitis is generally a clinical diagnosis with imaging limited to a confirmatory upright lateral plain film (Fig. 14-20). The patients often have increased stridor when they are placed supine; therefore cross-sectional imaging is not recommended—unless you have a gutsy radiologist. Furthermore, you want to keep the patient in a location close to where an emergency tracheostomy can be performed, which is usually not in an outpatient imaging center.
Pharyngoceles and Laryngoceles
Occasionally, a laryngocele may arise because of neoplastic processes (Fig. 14-21). To exclude a carcinoma at the saccule, endoscopy to evaluate the ventricle is recommended in patients with laryngoceles. Imaging studies should allow careful scrutiny of lesions in this region, which may be blind to endoscopy because of the overhanging shelf of the false cord.
Ranula
A final inflammatory lesion of the oral cavity is the ranula (mucus escape cyst). This is a cystic lesion that is due to obstruction of the sublingual gland duct or minor salivary gland of the floor of the mouth, which causes a backup of secretions. The obstruction may be due to previous infection, trauma, or calculi. Depending on whether the ranula protrudes through the mylohyoid musculature, the lesion may be termed simple (above the mylohyoid) or plunging (through the muscle). Do not pooh-pooh this minutia because it makes a difference to the surgeon—the simple ranula is approached via an intraoral resection, whereas the plunging ranula requires a submandibular or transcervical or combined intraoral and cervical resection. The ranula’s wall usually enhances. The ranula may have a pointed edge along its anterior extent in the sublingual space at the site of obstruction (Fig. 14-22). Rupture of a ranula can cause an encapsulated mucus-containing infection in the deep tissue of the neck.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


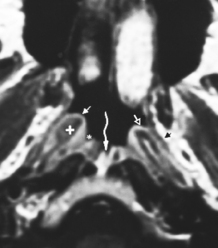
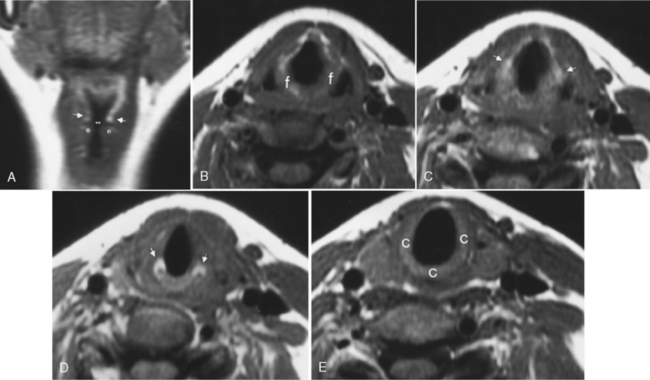
 ) from the thyroarytenoid muscle, which makes up the bulk of the true cord. One centimeter below the ventricle is the margin of the subglottic region. B, Axial T1WI demonstrates the supraglottic structures, including the aryepiglottic fold (f) extending posteroinferiorly toward the arytenoid region. C, At the false cord level one again can identify the fat (arrows) in the paraglottic space. D, At the true cord level the paraglottic tissue is made up of the thyroarytenoid musculature. The landmark for the true cord level is the cricoarytenoid joint (arrows). E, Subglottic region is marked by the full cricoid (c) ring.
) from the thyroarytenoid muscle, which makes up the bulk of the true cord. One centimeter below the ventricle is the margin of the subglottic region. B, Axial T1WI demonstrates the supraglottic structures, including the aryepiglottic fold (f) extending posteroinferiorly toward the arytenoid region. C, At the false cord level one again can identify the fat (arrows) in the paraglottic space. D, At the true cord level the paraglottic tissue is made up of the thyroarytenoid musculature. The landmark for the true cord level is the cricoarytenoid joint (arrows). E, Subglottic region is marked by the full cricoid (c) ring.