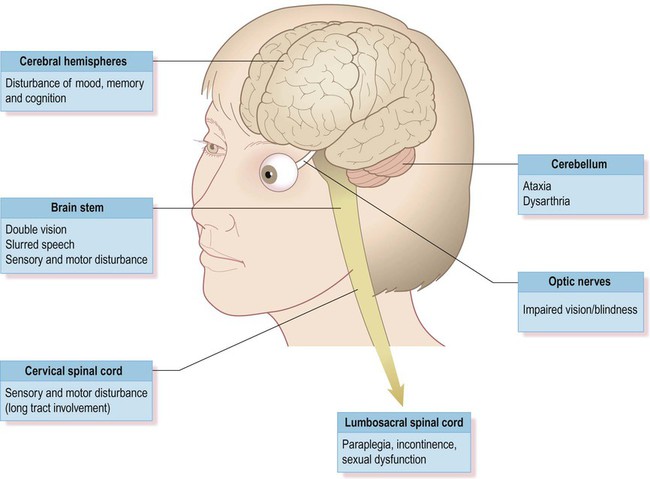
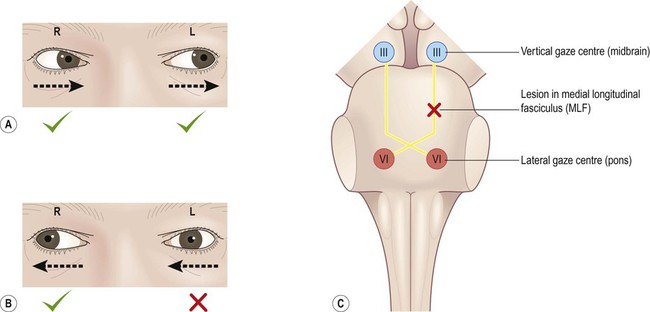
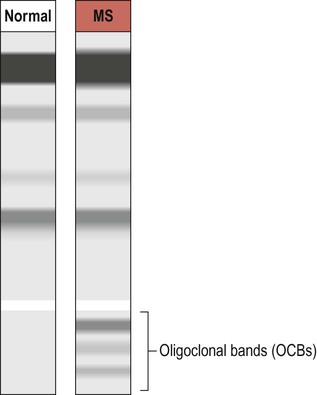
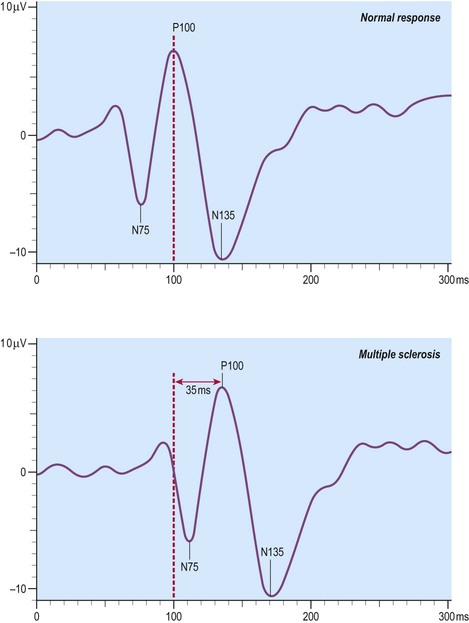
Axonal myelination is discussed in Chapter 5. The term demyelination refers to the loss of normally formed myelin and can be classified as primary or secondary: It is important to distinguish demyelination (loss of structurally normal myelin) from dysmyelination in which the myelin sheath is not normally formed in the first place. Conditions characterized by dysmyelination are usually due to a metabolic abnormality or enzyme deficiency and are often inherited (Clinical Box 14.1). Although plaques can occur anywhere in the brain or spinal cord, including the central visual pathways, some sites are more likely to be affected than others. This means that certain symptoms and signs are more common (Fig. 14.1). The most frequently encountered presenting features are weakness in one or more limbs (40% of cases) and optic neuritis (up to 25% of cases; discussed below). Inflammatory demyelination of the optic nerve (termed optic neuritis) is common in MS. This causes blurred vision in one eye, with reduced light and colour perception, combined with retrobulbar pain (discomfort behind the affected eye, exacerbated by movement). In some cases there is a blind spot or scotoma (Greek: scotos, darkness). Symptoms usually resolve completely within a few weeks, but there may be a persistent afferent pupillary defect (Clinical Box 14.2). Optic neuritis can occur as an isolated phenomenon, but 75% of affected individuals will eventually develop multiple sclerosis. Another common visual problem in MS is discussed in Clinical Box 14.3. Cognitive, emotional and behavioural changes occur in at least 40% of patients with MS. There may be subtle disturbances in frontal executive function (e.g. attention, working memory, decision-making; see Ch. 3) and a small proportion of patients develop more severe cognitive decline or even dementia (Ch. 12). Euphoria is often described, but depression is more common (seen in up to 50% of patients) and the risk of suicide is also increased. Psychotic features (delusions and hallucinations) are rare. Involvement of the cerebellum or its connections with the brain stem may cause dysarthria (slurred speech), ataxia (incoordination) or nystagmus (a rhythmic abnormality of gaze fixation, with a frequency of 1–4 Hz, consisting of a slow drift phase and a brisk corrective ‘snap’). There may also be a cerebellar intention tremor. This is worse towards the end of deliberate or precise movements (in contrast to the ‘rest tremor’ of Parkinson’s disease; see Ch. 13). Some MS symptoms are exacerbated (or clinically silent lesions unmasked) by an increase in body temperature. This can occur in a number of situations (e.g. a fever, hot bath or vigorous exercise) and is known as Uhthoff’s phenomenon. It is thought that increased temperature prolongs inactivation of voltage-gated sodium channels (see Ch. 6) and therefore increases the chance of conduction failure in partially myelinated or incompletely remyelinated axons. Demyelinating lesions are well-demonstrated on T2-weighted MRI scans, which highlight increased water content or decreased myelin (fat) content. However, since MS plaques tend to be periventricular, the T2 hyperintensity of normal CSF may make them more difficult to see. This is overcome using a fluid attenuation inversion recovery (FLAIR) sequence, which is similar to T2 but with a suppressed CSF signal (Fig. 14.4). In patients with clinically definite multiple sclerosis, MRI shows multifocal white matter abnormalities in 95% of cases. Administration of the MRI contrast agent gadolinium is useful for demonstrating acute (active) lesions. This correlates with breakdown of the blood–brain barrier (see Ch. 5) in areas of active inflammation and demyelination. The CNS inflammatory response in multiple sclerosis is associated with synthesis of antibodies (immunoglobulins) in the brain and spinal cord. It is therefore possible to detect antibodies in the CSF that are not present in peripheral blood. A sample of CSF is obtained by lumbar puncture (see Ch. 1, Clinical Box 1.3) and a specimen of venous blood is taken at the same time, for comparison. The two specimens are run on an electrophoretic gel to look for bands indicating the presence of type G immunoglobulins (IgG) that are only present in the CSF (which is indicative of CNS inflammation). These are known as oligoclonal bands (OCBs) and are found in 90% of people with MS (Fig. 14.5). Decreased conduction speed in the central visual pathways can be demonstrated in the majority of patients with MS by obtaining visual evoked potentials (VEPs). Scalp electrodes record electrical activity in the occipital cortex in response to a changing visual stimulus such as an alternating chequerboard pattern. The stimulus-response sequence is repeated many times and averaged (to increase the signal-to-noise ratio). This reveals a characteristic positive wave in the visual cortex at 100 milliseconds (the P100 wave) which is delayed by 30–40 milliseconds in 95% of people with MS (Fig. 14.6). This is a monoclonal antibody (immunoglobulin G, IgG) which is given by intravenous injection every 28 days. Clinical trials show that it reduces the number of relapses by about two-thirds. Natalizumab recognizes an adhesion molecule called α4 integrin which binds to a vascular cell adhesion molecule (VCAM-1) on endothelial cells. This is designed to prevent leukocytes from binding to blood vessels, reducing the number of chronic inflammatory cells entering the CNS from the bloodstream. Side effects include headache, nausea, vomiting and skin rash. In rare cases it has been associated with an acute white matter disorder: progressive multifocal leukoencephalopathy (PML) (Clinical Box 14.4).
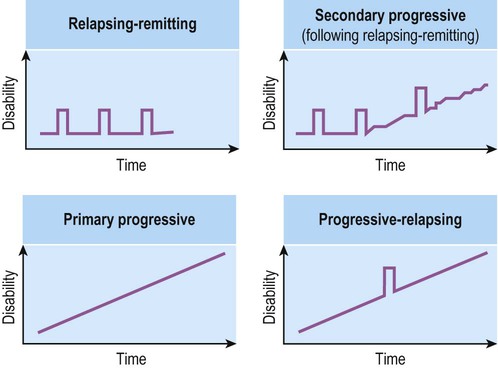
Multiple sclerosis
Demyelination
Clinical features of MS
Common symptoms
Loss of vision
Cognitive and emotional changes
Cerebellar features
Temperature sensitivity
Diagnosis and management
Diagnosis
Neuroimaging
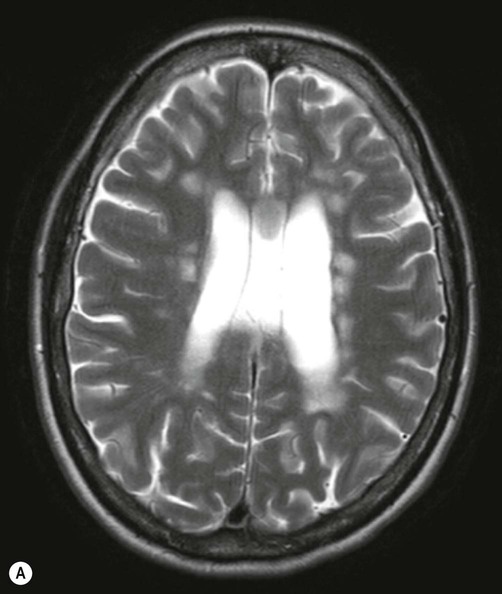
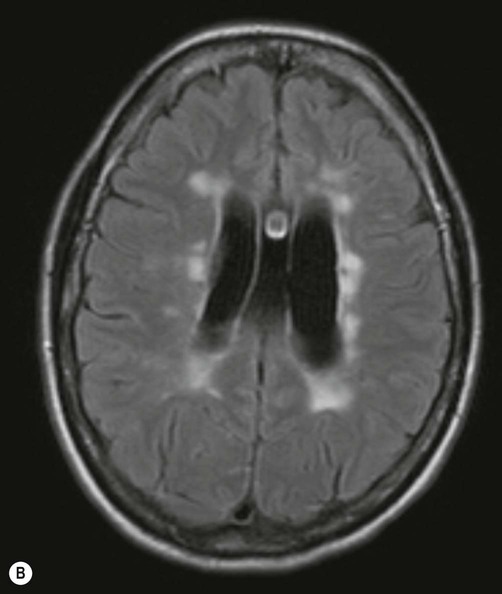
There are numerous plaque-like lesions in the periventricular white matter, best seen on the FLAIR sequence [see text for explanation]. Courtesy of Dr Andrew MacKinnon.
Oligoclonal bands
Visual evoked potentials
Management
Disease-modifying drugs (DMDs)
Natalizumab
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Multiple sclerosis
Only gold members can continue reading. Log In or Register to continue